An Fc-Optimized CD133 Antibody for Induction of Natural Killer Cell Reactivity against Colorectal Cancer
- PMID: 31181683
- PMCID: PMC6627285
- DOI: 10.3390/cancers11060789
An Fc-Optimized CD133 Antibody for Induction of Natural Killer Cell Reactivity against Colorectal Cancer
Abstract
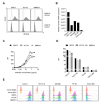
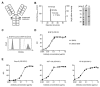
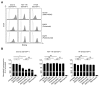
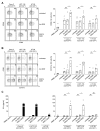
The introduction of monoclonal antibodies (mAbs) has largely improved treatment options for cancer patients. The ability of antitumor mAbs to elicit antibody-dependent cellular cytotoxicity (ADCC) contributes to a large extent to their therapeutic efficacy. Many efforts accordingly aim to improve this important function by engineering mAbs with Fc parts that display enhanced affinity to the Fc receptor CD16 expressed, e.g., on natural killer (NK) cells. Here we characterized the CD133 mAb 293C3-SDIE that contains an engineered Fc part modified by the amino acid exchanges S239D/I332E-that reportedly increase the affinity to CD16-with regard to its ability to induce NK reactivity against colorectal cancer (CRC). 293C3-SDIE was found to be a stable protein with favorable binding characteristics achieving saturating binding to CRC cells at concentrations of approximately 1 µg/mL. While not directly affecting CRC cell growth and viability, 293C3-SDIE potently induced NK cell activation, degranulation, secretion of Interferon-γ, as well as ADCC resulting in potent lysis of CRC cell lines. Based on the preclinical characterization presented in this study and the available data indicating that CD133 is broadly expressed in CRC and represents a negative prognostic marker, we conclude that 293C3-SDIE constitutes a promising therapeutic agent for the treatment of CRC and thus warrants clinical evaluation.
Keywords: ADCC; CD133; NK cells; antibody; colorectal cancer; immunotherapy; prominin-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
An Fc-optimized CD133 antibody for induction of NK cell reactivity against myeloid leukemia.Leukemia. 2017 Feb;31(2):459-469. doi: 10.1038/leu.2016.194. Epub 2016 Jul 20. Leukemia. 2017. PMID: 27435001
-
An Fc-Optimized CD133 Antibody for Induction of NK Cell Reactivity against B Cell Acute Lymphoblastic Leukemia.Cancers (Basel). 2021 Apr 1;13(7):1632. doi: 10.3390/cancers13071632. Cancers (Basel). 2021. PMID: 33915811 Free PMC article.
-
An Fc-modified monoclonal antibody as novel treatment option for pancreatic cancer.Front Immunol. 2024 Jan 22;15:1343929. doi: 10.3389/fimmu.2024.1343929. eCollection 2024. Front Immunol. 2024. PMID: 38322253 Free PMC article.
-
Natural Killer (NK) Cell Education Differentially Influences HIV Antibody-Dependent NK Cell Activation and Antibody-Dependent Cellular Cytotoxicity.Front Immunol. 2017 Aug 24;8:1033. doi: 10.3389/fimmu.2017.01033. eCollection 2017. Front Immunol. 2017. PMID: 28883824 Free PMC article. Review.
-
Combination therapy with interleukin-2 and antitumor monoclonal antibodies.Cancer J Sci Am. 1997 Dec;3 Suppl 1:S121-7. Cancer J Sci Am. 1997. PMID: 9457407 Review.
Cited by
-
B7-H3-targeting Fc-optimized antibody for induction of NK cell reactivity against sarcoma.Front Immunol. 2022 Oct 7;13:1002898. doi: 10.3389/fimmu.2022.1002898. eCollection 2022. Front Immunol. 2022. PMID: 36275693 Free PMC article.
-
KCNJ14 knockdown significantly inhibited the proliferation and migration of colorectal cells.BMC Med Genomics. 2022 Sep 13;15(1):194. doi: 10.1186/s12920-022-01351-4. BMC Med Genomics. 2022. PMID: 36100894 Free PMC article.
-
The War Is on: The Immune System against Glioblastoma-How Can NK Cells Drive This Battle?Biomedicines. 2022 Feb 8;10(2):400. doi: 10.3390/biomedicines10020400. Biomedicines. 2022. PMID: 35203609 Free PMC article. Review.
-
Unleashing Natural Killer Cells in the Tumor Microenvironment-The Next Generation of Immunotherapy?Front Immunol. 2020 Feb 21;11:275. doi: 10.3389/fimmu.2020.00275. eCollection 2020. Front Immunol. 2020. PMID: 32153582 Free PMC article. Review.
-
Immunomodulatory Role of NK Cells during Antiviral Antibody Therapy.Vaccines (Basel). 2021 Feb 8;9(2):137. doi: 10.3390/vaccines9020137. Vaccines (Basel). 2021. PMID: 33567792 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

