Selective use of primate CD4 receptors by HIV-1
- PMID: 31181085
- PMCID: PMC6586362
- DOI: 10.1371/journal.pbio.3000304
Selective use of primate CD4 receptors by HIV-1
Abstract
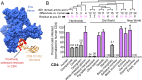
Individuals chronically infected with HIV-1 harbor complex viral populations within their bloodstreams. Recently, it has come to light that when these people infect others, the new infection is typically established by only one or a small number of virions from within this complex viral swarm. An important goal is to characterize the biological properties of HIV-1 virions that seed and exist early in new human infections because these are potentially the only viruses against which a prophylactic HIV-1 vaccine would need to elicit protection. This includes understanding how the Envelope (Env) protein of these virions interacts with the T-cell receptor CD4, which supports attachment and entry of HIV-1 into target cells. We examined early HIV-1 isolates for their ability to infect cells via the CD4 receptor of 15 different primate species. Primates were the original source of HIV-1 and now serve as valuable animal models for studying HIV-1. We find that most primary isolates of HIV-1 from the blood, including early isolates, are highly selective and enter cells through some primate CD4 receptor orthologs but not others. This phenotype is remarkably consistent, regardless of route of transmission, viral subtype, or time of isolation post infection. We show that the weak CD4 binding affinity of blood-derived HIV-1 isolates is what makes them sensitive to the small sequence differences in CD4 from one primate species to the next. To substantiate this, we engineered an early HIV-1 Env to have high, medium, or low binding affinity to CD4, and we show that it loses the ability to enter cells via the CD4 receptor of many primate species as the binding affinity gets weaker. Based on the phenotype of selective use of primate CD4, we find that weak CD4 binding appears to be a nearly universal property of HIV-1 circulating in the bloodstream. Therefore, weak binding to CD4 must be a selected and important property in the biology of HIV-1 in the body. We identify six primate species that encode CD4 receptors that fully support the entry of early HIV-1 isolates despite their low binding affinity for CD4. These findings will help inform long-standing efforts to model HIV-1 transmission and early disease in primates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
HIV-1 R5 Macrophage-Tropic Envelope Glycoprotein Trimers Bind CD4 with High Affinity, while the CD4 Binding Site on Non-macrophage-tropic, T-Tropic R5 Envelopes Is Occluded.J Virol. 2018 Jan 2;92(2):e00841-17. doi: 10.1128/JVI.00841-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29118121 Free PMC article.
-
A species-specific amino acid difference in the macaque CD4 receptor restricts replication by global circulating HIV-1 variants representing viruses from recent infection.J Virol. 2012 Dec;86(23):12472-83. doi: 10.1128/JVI.02176-12. Epub 2012 Sep 12. J Virol. 2012. PMID: 22973036 Free PMC article.
-
B cell recognition of the conserved HIV-1 co-receptor binding site is altered by endogenous primate CD4.PLoS Pathog. 2008 Oct 3;4(10):e1000171. doi: 10.1371/journal.ppat.1000171. PLoS Pathog. 2008. PMID: 18833294 Free PMC article.
-
Conformation-Dependent Interactions Between HIV-1 Envelope Glycoproteins and Broadly Neutralizing Antibodies.AIDS Res Hum Retroviruses. 2018 Sep;34(9):794-803. doi: 10.1089/AID.2018.0102. Epub 2018 Jul 17. AIDS Res Hum Retroviruses. 2018. PMID: 29905080 Review.
-
Unlocking HIV-1 Env: implications for antibody attack.AIDS Res Ther. 2017 Sep 12;14(1):42. doi: 10.1186/s12981-017-0168-5. AIDS Res Ther. 2017. PMID: 28893275 Free PMC article. Review.
Cited by
-
Conformation of HIV-1 Envelope Governs Rhesus CD4 Usage and Simian-Human Immunodeficiency Virus Replication.mBio. 2022 Feb 22;13(1):e0275221. doi: 10.1128/mbio.02752-21. Epub 2022 Jan 11. mBio. 2022. PMID: 35012342 Free PMC article.
-
Minimally Modified HIV-1 Infection of Macaques: Development, Utility, and Limitations of Current Models.Viruses. 2024 Oct 16;16(10):1618. doi: 10.3390/v16101618. Viruses. 2024. PMID: 39459950 Free PMC article. Review.
-
New SHIVs and Improved Design Strategy for Modeling HIV-1 Transmission, Immunopathogenesis, Prevention and Cure.J Virol. 2021 May 10;95(11):e00071-21. doi: 10.1128/JVI.00071-21. Epub 2021 Mar 3. J Virol. 2021. PMID: 33658341 Free PMC article.
-
Primate hemorrhagic fever-causing arteriviruses are poised for spillover to humans.Cell. 2022 Oct 13;185(21):3980-3991.e18. doi: 10.1016/j.cell.2022.09.022. Epub 2022 Sep 30. Cell. 2022. PMID: 36182704 Free PMC article.
-
Identification of HIV-1 Envelope Mutations that Enhance Entry Using Macaque CD4 and CCR5.Viruses. 2020 Feb 21;12(2):241. doi: 10.3390/v12020241. Viruses. 2020. PMID: 32098152 Free PMC article.
References
-
- Abrahams MR, Anderson JA, Giorgi EE, Seoighe C, Mlisana K, Ping LH, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol. 2009; 83: 3556–3567. 10.1128/JVI.02132-08 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

