Overexpression of GATA2 Enhances Development and Maintenance of Human Embryonic Stem Cell-Derived Hematopoietic Stem Cell-like Progenitors
- PMID: 31178416
- PMCID: PMC6626852
- DOI: 10.1016/j.stemcr.2019.05.007
Overexpression of GATA2 Enhances Development and Maintenance of Human Embryonic Stem Cell-Derived Hematopoietic Stem Cell-like Progenitors
Abstract
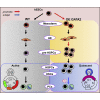
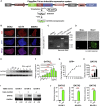
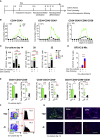
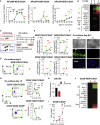
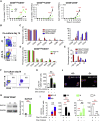
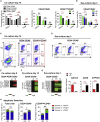
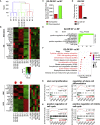
GATA2 is essential for the endothelial-to-hematopoietic transition (EHT) and generation of hematopoietic stem cells (HSCs). It is poorly understood how GATA2 controls the development of human pluripotent stem cell (hPSC)-derived HS-like cells. Here, using human embryonic stem cells (hESCs) in which GATA2 overexpression was induced by doxycycline (Dox), we elucidated the dual functions of GATA2 in definitive hematopoiesis before and after the emergence of CD34+CD45+CD90+CD38- HS-like cells. Specifically, GATA2 promoted expansion of hemogenic precursors via the EHT and then helped to maintain HS-like cells in a quiescent state by regulating cell cycle. RNA sequencing showed that hPSC-derived HS-like cells were very similar to human fetal liver-derived HSCs. Our findings will help to elucidate the mechanism that controls the early stages of human definitive hematopoiesis and may help to develop a strategy to generate hPSC-derived HSCs.
Keywords: GATA2; cell cycle; definitive hematopoiesis; hESCs; hematopoietic stem cells.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
GATA2 Is Dispensable for Specification of Hemogenic Endothelium but Promotes Endothelial-to-Hematopoietic Transition.Stem Cell Reports. 2018 Jul 10;11(1):197-211. doi: 10.1016/j.stemcr.2018.05.002. Epub 2018 May 31. Stem Cell Reports. 2018. PMID: 29861167 Free PMC article.
-
Functional and molecular characterization of mouse Gata2-independent hematopoietic progenitors.Blood. 2016 Mar 17;127(11):1426-37. doi: 10.1182/blood-2015-10-673749. Epub 2016 Feb 1. Blood. 2016. PMID: 26834239 Free PMC article.
-
Domain-specific biological functions of the transcription factor Gata2 on hematopoietic differentiation of mouse embryonic stem cells.Genes Cells. 2018 Sep;23(9):753-766. doi: 10.1111/gtc.12628. Epub 2018 Aug 8. Genes Cells. 2018. PMID: 30088690
-
The role of the GATA2 transcription factor in normal and malignant hematopoiesis.Crit Rev Oncol Hematol. 2012 Apr;82(1):1-17. doi: 10.1016/j.critrevonc.2011.04.007. Epub 2011 May 24. Crit Rev Oncol Hematol. 2012. PMID: 21605981 Review.
-
Dynamic regulation of GATA2 in fate determination in hematopoiesis: possible approach to hPSC-derived hematopoietic stem/progenitor cells.Blood Sci. 2020 Jan 16;2(1):1-6. doi: 10.1097/BS9.0000000000000040. eCollection 2020 Jan. Blood Sci. 2020. PMID: 35399862 Free PMC article. Review.
Cited by
-
HOXC4 up-regulates NF-κB signaling and promotes the cell proliferation to drive development of human hematopoiesis, especially CD43+ cells.Blood Sci. 2020 Sep 1;2(4):117-128. doi: 10.1097/BS9.0000000000000054. eCollection 2020 Oct. Blood Sci. 2020. PMID: 35400027 Free PMC article.
-
Exploring the Molecular and Developmental Dynamics of Endothelial Cell Differentiation.Int J Stem Cells. 2024 Feb 28;17(1):15-29. doi: 10.15283/ijsc23086. Epub 2023 Oct 26. Int J Stem Cells. 2024. PMID: 37879853 Free PMC article. Review.
-
Overexpression of p21 Has Inhibitory Effect on Human Hematopoiesis by Blocking Generation of CD43+ Cells via Cell-Cycle Regulation.Int J Stem Cells. 2020 Jul 30;13(2):202-211. doi: 10.15283/ijsc20033. Int J Stem Cells. 2020. PMID: 32587134 Free PMC article.
-
Multifaceted Actions of GFI1 and GFI1B in Hematopoietic Stem Cell Self-Renewal and Lineage Commitment.Front Genet. 2020 Oct 26;11:591099. doi: 10.3389/fgene.2020.591099. eCollection 2020. Front Genet. 2020. PMID: 33193732 Free PMC article. Review.
-
Ventral tegmental area dopaminergic action in music therapy for post-traumatic stress disorder: A literature review.Front Psychol. 2022 Oct 10;13:1014202. doi: 10.3389/fpsyg.2022.1014202. eCollection 2022. Front Psychol. 2022. PMID: 36300072 Free PMC article. Review.
References
-
- Böiers C., Carrelha J., Lutteropp M., Luc S., Green J.C., Azzoni E., Woll P.S., Mead A.J., Hultquist A., Swiers G. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13:535–548. - PubMed
-
- Boisset J.C., van Cappellen W., Andrieu-Soler C., Galjart N., Dzierzak E., Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. - PubMed
-
- Chen B., Teng J., Liu H., Pan X., Zhou Y., Huang S., Lai M., Bian G., Mao B., Sun W. Inducible overexpression of RUNX1b/c in human embryonic stem cells blocks early hematopoiesis from mesoderm. J. Mol. Cell Biol. 2017;9:262–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

