A Biased View of μ-Opioid Receptors?
- PMID: 31175184
- PMCID: PMC6784500
- DOI: 10.1124/mol.119.115956
A Biased View of μ-Opioid Receptors?
Abstract
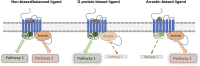
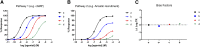
The field of biased agonism has grown substantially in recent years and the μ-opioid receptor has been one of the most intensively studied receptor targets for developing biased agonists. Yet, despite extensive research efforts, the development of analgesics with reduced adverse effects remains a significant challenge. In this review we discuss the evidence to support the prevailing hypothesis that a G protein-biased agonist at the μ-opioid receptor would be an effective analgesic without the accompanying adverse effects associated with conventional μ-opioid agonists. We also assess the current status of established and novel μ-opioid-receptor ligands that are proposed to be biased ligands. SIGNIFICANCE STATEMENT: The idea that biased agonists at the μ-opioid receptor might provide a therapeutic advantage in terms of producing effective analgesia with fewer adverse effects has driven the design of novel G protein-biased agonists. However, is the desirability of G protein-biased agonists at μ-opioid receptor substantiated by what we know of the physiology and pharmacology of the receptor? Also, do any of the novel biased agonists live up to their initial promise? Here we address these issues by critically examining the evidence that G protein bias really is desirable and also by discussing whether the ligands so far developed are clearly biased in vitro and whether this produces responses in vivo that might be commensurate with such bias.
Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
Similar articles
-
Mu Opioids Induce Biased Signaling at the Full-Length Seven Transmembrane C-Terminal Splice Variants of the mu Opioid Receptor Gene, Oprm1.Cell Mol Neurobiol. 2021 Jul;41(5):1059-1074. doi: 10.1007/s10571-020-00973-5. Epub 2020 Oct 8. Cell Mol Neurobiol. 2021. PMID: 33033993 Free PMC article.
-
Biased agonism of clinically approved μ-opioid receptor agonists and TRV130 is not controlled by binding and signaling kinetics.Neuropharmacology. 2020 Apr;166:107718. doi: 10.1016/j.neuropharm.2019.107718. Epub 2019 Jul 24. Neuropharmacology. 2020. PMID: 31351108
-
Mu-Opioid receptor biased ligands: A safer and painless discovery of analgesics?Drug Discov Today. 2017 Nov;22(11):1719-1729. doi: 10.1016/j.drudis.2017.07.002. Epub 2017 Jul 22. Drug Discov Today. 2017. PMID: 28743488 Free PMC article. Review.
-
Biased mu-opioid receptor ligands: a promising new generation of pain therapeutics.Curr Opin Pharmacol. 2017 Feb;32:77-84. doi: 10.1016/j.coph.2016.11.007. Epub 2016 Dec 7. Curr Opin Pharmacol. 2017. PMID: 27936408 Review.
-
Synthesis, biological, and structural explorations of a series of μ-opioid receptor (MOR) agonists with high G protein signaling bias.Eur J Med Chem. 2022 Jan 15;228:113986. doi: 10.1016/j.ejmech.2021.113986. Epub 2021 Nov 12. Eur J Med Chem. 2022. PMID: 34802839
Cited by
-
Addressing opioid tolerance and opioid-induced hypersensitivity: Recent developments and future therapeutic strategies.Pharmacol Res Perspect. 2021 May;9(3):e00789. doi: 10.1002/prp2.789. Pharmacol Res Perspect. 2021. PMID: 34096178 Free PMC article. Review.
-
Mind the Gap-Deciphering GPCR Pharmacology Using 3D Pharmacophores and Artificial Intelligence.Pharmaceuticals (Basel). 2022 Oct 22;15(11):1304. doi: 10.3390/ph15111304. Pharmaceuticals (Basel). 2022. PMID: 36355476 Free PMC article. Review.
-
Confronting the opioid crisis with basic research in neuropharmacology.Neuropharmacology. 2020 Apr;166:107972. doi: 10.1016/j.neuropharm.2020.107972. Epub 2020 Jan 17. Neuropharmacology. 2020. PMID: 31958407 Free PMC article. No abstract available.
-
Site selective C-H functionalization of Mitragyna alkaloids reveals a molecular switch for tuning opioid receptor signaling efficacy.Nat Commun. 2021 Jun 22;12(1):3858. doi: 10.1038/s41467-021-23736-2. Nat Commun. 2021. PMID: 34158473 Free PMC article.
-
Pharmacological Characterization of µ-Opioid Receptor Agonists with Biased G Protein or β-Arrestin Signaling, and Computational Study of Conformational Changes during Receptor Activation.Molecules. 2020 Dec 22;26(1):13. doi: 10.3390/molecules26010013. Molecules. 2020. PMID: 33375124 Free PMC article.
References
-
- Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS. (2017) Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol 31:730–739. - PMC - PubMed
-
- Black JW, Leff P. (1983) Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci 220:141–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

