Development of an intravaginal ring delivering simultaneously anastrozole and levonorgestrel: a pharmacokinetic perspective
- PMID: 31174438
- PMCID: PMC6567139
- DOI: 10.1080/10717544.2019.1622609
Development of an intravaginal ring delivering simultaneously anastrozole and levonorgestrel: a pharmacokinetic perspective
Abstract
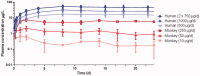
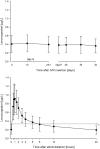
Intravaginal rings (IVRs) are an option for continuous administration of drugs in women. As an attractive approach for the treatment of endometriosis-associated pelvic pain, IVRs delivering a combination of the aromatase inhibitor anastrozole (ATZ) and the progestin levonorgestrel (LNG) have been developed. This article describes the developmental pharmacokinetic (PK) aspects covering the characterization of in vitro release, preclinical IVR PK investigations in monkeys, and clinical PK considerations. An IVR for ATZ has been developed and investigated in healthy menstruating female cynomolgus monkeys showing effective in vivo release. PK data from the size-adapted IVR used in these animals can be translated into a human context as confirmed in human studies where predefined exposure levels of ATZ were reached. As ATZ may cause harm to the fetus, use of effective contraception has to be assured in women of childbearing potential. Therefore, the IVR delivers a low dose of LNG as a contraceptive. Although the daily dose differed strongly between both drugs (20 µg LNG/d to >1 mg ATZ/d), simultaneous delivery of ATZ and LNG in vitro and in vivo was observed with a high correlation between the in vitro release and PK profiles. The PK characteristics successfully guided the design of clinical studies investigating the drug-drug interaction (DDI) potential. No relevant DDI between both the investigated or other vaginally administered drugs were identified.
Keywords: Clinical pharmacokinetics; drug delivery; gynecology; women's health.
Figures

Similar articles
-
Absence of Drug-Drug Interaction of Anastrozole on Levonorgestrel Delivered Simultaneously by an Intravaginal Ring: Results of a Phase 2 Trial.J Clin Pharmacol. 2019 Jul;59(7):1022-1028. doi: 10.1002/jcph.1396. Epub 2019 Feb 21. J Clin Pharmacol. 2019. PMID: 30791125 Free PMC article. Clinical Trial.
-
Pharmacokinetics, pharmacodynamics, safety and tolerability of an intravaginal ring releasing anastrozole and levonorgestrel in healthy premenopausal women: a Phase 1 randomized controlled trial.Hum Reprod. 2016 Aug;31(8):1713-22. doi: 10.1093/humrep/dew145. Epub 2016 Jun 19. Hum Reprod. 2016. PMID: 27390369 Clinical Trial.
-
Characterization of anastrozole effects, delivered by an intravaginal ring in cynomolgus monkeys.Hum Reprod. 2015 Feb;30(2):308-14. doi: 10.1093/humrep/deu315. Epub 2014 Nov 28. Hum Reprod. 2015. PMID: 25432919
-
A risk-benefit assessment of the levonorgestrel-releasing intrauterine system.Drug Saf. 1996 Dec;15(6):430-40. doi: 10.2165/00002018-199615060-00006. Drug Saf. 1996. PMID: 8968696 Review.
-
New knowledge in the physiology of hormonal contraceptives.Am J Obstet Gynecol. 1994 May;170(5 Pt 2):1499-507. doi: 10.1016/s0002-9378(94)05011-8. Am J Obstet Gynecol. 1994. PMID: 8178898 Review.
Cited by
-
A Systematic Review of Systematic Reviews on the Use of Aromatase Inhibitors for the Treatment of Endometriosis: The Evidence to Date.Drug Des Devel Ther. 2023 May 4;17:1329-1346. doi: 10.2147/DDDT.S315726. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37168488 Free PMC article. Review.
References
-
- Alexander NJ, Baker E, Kaptein M, et al. (2004). Why consider vaginal drug administration? Fertil Steril 82:1–12. - PubMed
-
- Amsterdam LL, Gentry W, Jobanputra S, et al. (2005). Anastrazole and oral contraceptives: a novel treatment for endometriosis. Fertil Steril 84:300–4. - PubMed
-
- Arimidex® (2017). Summary of product characteristics. Available at: https://www.medicines.org.uk/emc/product/2199/smpc [last accessed 12 Feb 2019]
-
- Ballagh SA. (2001). Vaginal ring hormone delivery systems in contraception and menopause. Clin Obstet Gynecol 44:106–13. - PubMed
-
- Bayer Jadelle® Prescribing information “Norplant II®” Levonorgestrel Implants (Jadelle®). NDA 20-544 Supplement 003 Prescribing information. 2002. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2002/20544se2-003_ja... [last accessed 12 Feb 2019]
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
