Constitutive inhibitory G protein activity upon adenylyl cyclase-dependent cardiac contractility is limited to adenylyl cyclase type 6
- PMID: 31173603
- PMCID: PMC6556981
- DOI: 10.1371/journal.pone.0218110
Constitutive inhibitory G protein activity upon adenylyl cyclase-dependent cardiac contractility is limited to adenylyl cyclase type 6
Abstract
Purpose: We previously reported that inhibitory G protein (Gi) exerts intrinsic receptor-independent inhibitory activity upon adenylyl cyclase (AC) that regulates contractile force in rat ventricle. The two major subtypes of AC in the heart are AC5 and AC6. The aim of this study was to determine if this intrinsic Gi inhibition regulating contractile force is AC subtype selective.
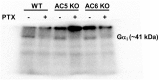
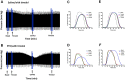
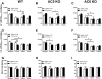
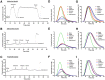
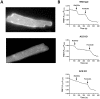
Methods: Wild-type (WT), AC5 knockout (AC5KO) and AC6 knockout (AC6KO) mice were injected with pertussis toxin (PTX) to inactivate Gi or saline (control).Three days after injection, we evaluated the effect of simultaneous inhibition of phosphodiesterases (PDE) 3 and 4 with cilostamide and rolipram respectively upon in vivo and ex vivo left ventricular (LV) contractile function. Also, changes in the level of cAMP were measured in left ventricular homogenates and at the membrane surface in cardiomyocytes obtained from the same mouse strains expressing the cAMP sensor pmEPAC1 using fluorescence resonance energy transfer (FRET).
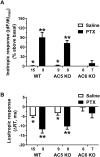
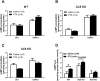
Results: Simultaneous PDE3 and PDE4 inhibition increased in vivo and ex vivo rate of LV contractility only in PTX-treated WT and AC5KO mice but not in saline-treated controls. Likewise, Simultaneous PDE3 and PDE4 inhibition elevated total cAMP levels in PTX-treated WT and AC5KO mice compared to saline-treated controls. In contrast, simultaneous PDE3 and PDE4 inhibition did not increase in vivo or ex vivo rate of LV contractility or cAMP levels in PTX-treated AC6KO mice compared to saline-treated controls. Using FRET analysis, an increase of cAMP level was detected at the membrane of cardiomyocytes after simultaneous PDE3 and PDE4 inhibition in WT and AC5KO but not AC6KO. These FRET data are consistent with the functional data indicating that AC6 activity and PTX inhibition of Gi is necessary for simultaneous inhibition of PDE3 and PDE4 to elicit an increase in contractility.
Conclusions: Together, these data suggest that AC6 is tightly regulated by intrinsic receptor-independent Gi activity, thus providing a mechanism for maintaining low basal cAMP levels in the functional compartment that regulates contractility.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Knockout of adenylyl cyclase isoform 5 or 6 differentially modifies the β1-adrenoceptor-mediated inotropic response.J Mol Cell Cardiol. 2019 Jun;131:132-145. doi: 10.1016/j.yjmcc.2019.04.017. Epub 2019 Apr 19. J Mol Cell Cardiol. 2019. PMID: 31009605
-
Gi proteins regulate adenylyl cyclase activity independent of receptor activation.PLoS One. 2014 Sep 9;9(9):e106608. doi: 10.1371/journal.pone.0106608. eCollection 2014. PLoS One. 2014. PMID: 25203113 Free PMC article.
-
The functional activity of inhibitory G protein (G(i)) is not increased in failing heart ventricle.J Mol Cell Cardiol. 2013 Mar;56:129-38. doi: 10.1016/j.yjmcc.2012.11.015. Epub 2012 Dec 7. J Mol Cell Cardiol. 2013. PMID: 23220156
-
The inhibitory G protein G(i) identified as pertussis toxin-catalyzed ADP-ribosylation.Biol Pharm Bull. 2012;35(12):2103-11. doi: 10.1248/bpb.b212024. Biol Pharm Bull. 2012. PMID: 23207763 Review.
-
Regulation of murine cardiac function by phosphodiesterases type 3 and 4.Curr Opin Pharmacol. 2011 Dec;11(6):714-9. doi: 10.1016/j.coph.2011.10.017. Epub 2011 Oct 31. Curr Opin Pharmacol. 2011. PMID: 22047792 Review.
Cited by
-
Physiological roles of mammalian transmembrane adenylyl cyclase isoforms.Physiol Rev. 2022 Apr 1;102(2):815-857. doi: 10.1152/physrev.00013.2021. Epub 2021 Oct 26. Physiol Rev. 2022. PMID: 34698552 Free PMC article. Review.
-
Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction.Cells. 2021 Apr 16;10(4):922. doi: 10.3390/cells10040922. Cells. 2021. PMID: 33923648 Free PMC article. Review.
References
-
- Smit MJ, Iyengar R. Mammalian adenylyl cyclases. Advances in second messenger and phosphoprotein research. 1998;32:1–21. Epub 1998/01/09. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

