HPLC-Based Monitoring of Oxidation of Hydroethidine for the Detection of NADPH Oxidase-Derived Superoxide Radical Anion
- PMID: 31172476
- PMCID: PMC6953631
- DOI: 10.1007/978-1-4939-9424-3_14
HPLC-Based Monitoring of Oxidation of Hydroethidine for the Detection of NADPH Oxidase-Derived Superoxide Radical Anion
Abstract
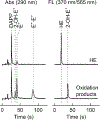
Hydroethidine is a fluorogenic probe that in the presence of the superoxide radical anion is oxidized to a red fluorescent product, 2-hydroxyethidium. In cells, hydroethidine is also oxidized to other products, including red fluorescent ethidium. Thus, selective monitoring of 2-hydroxyethidium is required for specific detection of the superoxide radical anion. Here, we provide protocols for HPLC- and LC-MS-based quantitation of 2-hydroxyethidium, among other oxidation products. Also, a protocol for continuous sampling for real-time monitoring of superoxide production using rapid HPLC measurements of 2-hydroxyethidium is described.
Keywords: 2-hydroxyethidium; HPLC; Hydroethidine; LC-MS; Superoxide radical anion.
Figures
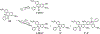





Similar articles
-
HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes--the ultimate approach for intra- and extracellular superoxide detection.Biochim Biophys Acta. 2014 Feb;1840(2):739-44. doi: 10.1016/j.bbagen.2013.05.008. Epub 2013 May 10. Biochim Biophys Acta. 2014. PMID: 23668959 Free PMC article. Review.
-
Measurement of Superoxide Production and NADPH Oxidase Activity by HPLC Analysis of Dihydroethidium Oxidation.Methods Mol Biol. 2017;1527:233-249. doi: 10.1007/978-1-4939-6625-7_19. Methods Mol Biol. 2017. PMID: 28116721
-
Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide.Free Radic Biol Med. 2003 Jun 1;34(11):1359-68. doi: 10.1016/s0891-5849(03)00142-4. Free Radic Biol Med. 2003. PMID: 12757846
-
HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements.Free Radic Biol Med. 2009 Feb 1;46(3):329-38. doi: 10.1016/j.freeradbiomed.2008.10.031. Epub 2008 Oct 29. Free Radic Biol Med. 2009. PMID: 19026738 Free PMC article.
-
Recent developments in detection of superoxide radical anion and hydrogen peroxide: Opportunities, challenges, and implications in redox signaling.Arch Biochem Biophys. 2017 Mar 1;617:38-47. doi: 10.1016/j.abb.2016.08.021. Epub 2016 Aug 30. Arch Biochem Biophys. 2017. PMID: 27590268 Free PMC article. Review.
Cited by
-
Mitochondrial dysfunction and metabolic reprogramming induce macrophage pro-inflammatory phenotype switch and atherosclerosis progression in aging.Front Immunol. 2024 Jun 21;15:1410832. doi: 10.3389/fimmu.2024.1410832. eCollection 2024. Front Immunol. 2024. PMID: 38975335 Free PMC article.
-
Health position paper and redox perspectives - Disease burden by transportation noise.Redox Biol. 2024 Feb;69:102995. doi: 10.1016/j.redox.2023.102995. Epub 2023 Dec 18. Redox Biol. 2024. PMID: 38142584 Free PMC article. Review.
-
Oxidation of ethidium-based probes by biological radicals: mechanism, kinetics and implications for the detection of superoxide.Sci Rep. 2020 Oct 29;10(1):18626. doi: 10.1038/s41598-020-75373-2. Sci Rep. 2020. PMID: 33122809 Free PMC article.
-
Nitric oxide synthase and reduced arterial tone contribute to arteriovenous malformation.Sci Adv. 2023 May 26;9(21):eade7280. doi: 10.1126/sciadv.ade7280. Epub 2023 May 26. Sci Adv. 2023. PMID: 37235659 Free PMC article.
-
Novel NADPH Oxidase-2 Inhibitors as Potential Anti-Inflammatory and Neuroprotective Agents.Antioxidants (Basel). 2023 Aug 23;12(9):1660. doi: 10.3390/antiox12091660. Antioxidants (Basel). 2023. PMID: 37759963 Free PMC article.
References
-
- Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4:181–189 - PubMed
-
- Winterbourn CC (2008) Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4:278–286 - PubMed
-
- Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313 - PubMed
-
- Maghzal GJ, Krause KH, Stocker R, Jaquet V (2012) Detection of reactive oxygen species derived from the family of NOX NADPH oxidases. Free Radic Biol Med 53:1903–1918 - PubMed
-
- Wardman P (2007) Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med 43:995–1022 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

