Comparison of the effect of growth factors on chondrogenesis of canine mesenchymal stem cells
- PMID: 31167981
- PMCID: PMC6715918
- DOI: 10.1292/jvms.18-0551
Comparison of the effect of growth factors on chondrogenesis of canine mesenchymal stem cells
Abstract
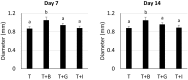
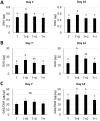
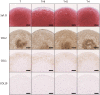
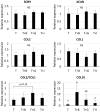
Mesenchymal stem cells (MSCs) are proposed to be useful in cartilage regenerative medicine, however, canine MSCs have been reported to show poor chondrogenic capacity. Therefore, optimal conditions for chondrogenic differentiation should be determined by mimicking the developmental process. We have previously established novel and superior canine MSCs named bone marrow peri-adipocyte cells (BM-PACs) and the objective of this study was to evaluate the effects of growth factors required for in vivo chondrogenesis using canine BM-PACs. Spheroids of BM-PACs were cultured in chondrogenic medium containing 10 ng/ml transforming growth factor-β1 (TGF-β1) with or without 100 ng/ml bone morphogenetic protein-2 (BMP-2), 100 ng/ml growth differentiation factor-5 (GDF-5) or 100 ng/ml insulin-like growth factor-1 (IGF-1). Chondrogenic differentiation was evaluated by the quantification of glycosaminoglycan and Safranin O staining for proteoglycan production. The expression of cartilage matrix or hypertrophic gene/protein was also evaluated by qPCR and immunohistochemistry. Spheroids in all groups were strongly stained with Safranin O. Although BMP-2 significantly increased glycosaminoglycan production, Safranin O-negative outer layer was formed and the mRNA expression of COL10 relating to cartilage hypertrophy was also significantly upregulated (P<0.05). GDF-5 promoted the production of glycosaminoglycan and type II collagen without increasing COL10 mRNA expression. The supplementation of IGF-1 did not significantly affect cartilaginous and hypertrophic differentiation. Our results indicate that GDF-5 is a useful growth factor for the generation of articular cartilage from canine MSCs.
Keywords: canine; cartilage; chondrogenesis; growth factor; mesenchymal stem cell.
Figures
Similar articles
-
Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells.J Cell Physiol. 2010 Apr;223(1):84-93. doi: 10.1002/jcp.22013. J Cell Physiol. 2010. PMID: 20049852
-
Chondrogenic differentiation of synovial fluid mesenchymal stem cells on human meniscus-derived decellularized matrix requires exogenous growth factors.Acta Biomater. 2018 Oct 15;80:131-143. doi: 10.1016/j.actbio.2018.09.038. Epub 2018 Sep 26. Acta Biomater. 2018. PMID: 30267878
-
Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling.J Bone Miner Res. 2006 Apr;21(4):626-36. doi: 10.1359/jbmr.051213. Epub 2006 Apr 5. J Bone Miner Res. 2006. PMID: 16598383
-
The role of growth factors in stem cell-directed chondrogenesis: a real hope for damaged cartilage regeneration.Int Orthop. 2015 May;39(5):995-1003. doi: 10.1007/s00264-014-2619-0. Epub 2014 Dec 16. Int Orthop. 2015. PMID: 25512139 Review.
-
Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering.Tissue Eng Part B Rev. 2009 Mar;15(1):29-41. doi: 10.1089/ten.teb.2008.0329. Tissue Eng Part B Rev. 2009. PMID: 19063663 Free PMC article. Review.
Cited by
-
Growth differentiation factor 5 in cartilage and osteoarthritis: A possible therapeutic candidate.Cell Prolif. 2021 Mar;54(3):e12998. doi: 10.1111/cpr.12998. Epub 2021 Feb 1. Cell Prolif. 2021. PMID: 33522652 Free PMC article. Review.
-
Plasma Glycosaminoglycans in Children with Juvenile Idiopathic Arthritis Being Treated with Etanercept as Potential Biomarkers of Joint Dysfunction.Biomedicines. 2022 Jul 31;10(8):1845. doi: 10.3390/biomedicines10081845. Biomedicines. 2022. PMID: 36009392 Free PMC article.
-
Simvastatin Enhances the Chondrogenesis But Not the Osteogenesis of Adipose-Derived Stem Cells in a Hyaluronan Microenvironment.Biomedicines. 2021 May 17;9(5):559. doi: 10.3390/biomedicines9050559. Biomedicines. 2021. PMID: 34067739 Free PMC article.
-
Substantial Overview on Mesenchymal Stem Cell Biological and Physical Properties as an Opportunity in Translational Medicine.Int J Mol Sci. 2019 Oct 29;20(21):5386. doi: 10.3390/ijms20215386. Int J Mol Sci. 2019. PMID: 31671788 Free PMC article. Review.
-
Microtissue Culture Provides Clarity on the Relative Chondrogenic and Hypertrophic Response of Bone-Marrow-Derived Stromal Cells to TGF-β1, BMP-2, and GDF-5.Cells. 2023 Dec 23;13(1):37. doi: 10.3390/cells13010037. Cells. 2023. PMID: 38201241 Free PMC article.
References
-
- Ayerst B. I., Smith R. A. A., Nurcombe V., Day A. J., Merry C. L. R., Cool S. M.2017. Growth differentiation factor 5-mediated enhancement of chondrocyte phenotype is inhibited by heparin: implications for the use of heparin in the clinic and in tissue engineering applications. Tissue Eng. Part A 23: 275–292. doi: 10.1089/ten.tea.2016.0364 - DOI - PMC - PubMed
-
- Bartunek J., Croissant J. D., Wijns W., Gofflot S., de Lavareille A., Vanderheyden M., Kaluzhny Y., Mazouz N., Willemsen P., Penicka M., Mathieu M., Homsy C., De Bruyne B., McEntee K., Lee I. W., Heyndrickx G. R.2007. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am. J. Physiol. Heart Circ. Physiol. 292: H1095–H1104. doi: 10.1152/ajpheart.01009.2005 - DOI - PubMed
-
- Bertolo, A., Schlaefli, P., Malonzo-Marty, C., Baur, M., Pötzel, T., Steffen, F, Stoyanov, J. 2015. Comparative characterization of canine and human mesenchymal stem cells derived from bone marrow. Int. J. Stem Cell Res. Ther. 2: 1–7.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

