Esculetin, a Coumarin Derivative, Prevents Thrombosis: Inhibitory Signaling on PLCγ2-PKC-AKT Activation in Human Platelets
- PMID: 31163690
- PMCID: PMC6600380
- DOI: 10.3390/ijms20112731
Esculetin, a Coumarin Derivative, Prevents Thrombosis: Inhibitory Signaling on PLCγ2-PKC-AKT Activation in Human Platelets
Abstract
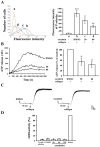
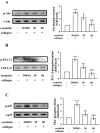
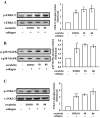
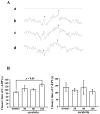
Esculetin, a bioactive 6,7-dihydroxy derivative of coumarin, possesses pharmacological activities against obesity, diabetes, renal failure, and cardiovascular disorders (CVDs). Platelet activation plays a major role in CVDs. Thus, disrupting platelet activation represents an attractive therapeutic target. We examined the effect of esculetin in human platelet activation and experimental mouse models. At 10-80 μM, esculetin inhibited collagen- and arachidonic acid-induced platelet aggregation in washed human platelets. However, it had no effects on other agonists such as thrombin and U46619. Esculetin inhibited adenosine triphosphate release, P-selectin expression, hydroxyl radical (OH·) formation, Akt activation, and phospholipase C (PLC)γ2/protein kinase C (PKC) phosphorylation, but did not diminish mitogen-activated protein kinase phosphorylation in collagen-activated human platelets. Platelet function analysis indicated that esculetin substantially prolonged the closure time of whole blood. In experimental mice, esculetin significantly increased the occlusion time in thrombotic platelet plug formation and reduced mortality associated with acute pulmonary thromboembolism. However, it did not prolong the bleeding time. This study demonstrates that esculetin inhibits human platelet activation via hindering the PLCγ2-PKC cascade, hydroxyl radical formation, Akt activation, and ultimately suppressing platelet activation. Therefore, esculetin may act as an essential therapeutic agent for preventing thromboembolic diseases.
Keywords: arterial thrombosis; esculetin; experimental mice; human platelets; hydroxyl radical; signaling pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Regulation of Key Antiplatelet Pathways by Bioactive Compounds with Minimal Bleeding Risk.Int J Mol Sci. 2021 Nov 17;22(22):12380. doi: 10.3390/ijms222212380. Int J Mol Sci. 2021. PMID: 34830261 Free PMC article. Review.
-
Hinokitiol inhibits platelet activation ex vivo and thrombus formation in vivo.Biochem Pharmacol. 2013 May 15;85(10):1478-85. doi: 10.1016/j.bcp.2013.02.027. Epub 2013 Mar 5. Biochem Pharmacol. 2013. PMID: 23473801
-
Glabridin, a Bioactive Flavonoid from Licorice, Effectively Inhibits Platelet Activation in Humans and Mice.Int J Mol Sci. 2022 Sep 27;23(19):11372. doi: 10.3390/ijms231911372. Int J Mol Sci. 2022. PMID: 36232674 Free PMC article.
-
Prevention of arterial thrombosis by nobiletin: in vitro and in vivo studies.J Nutr Biochem. 2016 Feb;28:1-8. doi: 10.1016/j.jnutbio.2015.09.024. Epub 2015 Oct 17. J Nutr Biochem. 2016. PMID: 26878777
-
Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules.Cardiovasc Toxicol. 2020 Feb;20(1):1-10. doi: 10.1007/s12012-019-09555-4. Cardiovasc Toxicol. 2020. PMID: 31784932 Review.
Cited by
-
Suppression of Human Platelet Activation via Integrin αIIbβ3 Outside-In Independent Signal and Reduction of the Mortality in Pulmonary Thrombosis by Auraptene.Int J Mol Sci. 2019 Nov 8;20(22):5585. doi: 10.3390/ijms20225585. Int J Mol Sci. 2019. PMID: 31717348 Free PMC article.
-
Regulation of Key Antiplatelet Pathways by Bioactive Compounds with Minimal Bleeding Risk.Int J Mol Sci. 2021 Nov 17;22(22):12380. doi: 10.3390/ijms222212380. Int J Mol Sci. 2021. PMID: 34830261 Free PMC article. Review.
-
Artemisia scoparia and Metabolic Health: Untapped Potential of an Ancient Remedy for Modern Use.Front Endocrinol (Lausanne). 2022 Feb 8;12:727061. doi: 10.3389/fendo.2021.727061. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35211087 Free PMC article. Review.
-
Antiplatelet Effect of Daphnetin Is Regulated by cPLA2-Mediated Thromboxane A2 Generation in Mice.Int J Mol Sci. 2023 Mar 17;24(6):5779. doi: 10.3390/ijms24065779. Int J Mol Sci. 2023. PMID: 36982853 Free PMC article.
-
Antioxidant and anti‑inflammatory effects of esculin and esculetin (Review).Exp Ther Med. 2024 Apr 12;27(6):248. doi: 10.3892/etm.2024.12536. eCollection 2024 Jun. Exp Ther Med. 2024. PMID: 38682114 Free PMC article. Review.
References
-
- Wang T.Y., White J.A., Tricoci P., Giugliano R.P., Zeymer U., Harrington R.A., Montalescot G., James S.K., Van de Werf F., Armstrong P.W., et al. Upstream clopidogrel use and the efficacy and safety of early eptifibatide treatment in patients with acute coronary syndrome: An analysis from the early glycoprotein IIb/IIIa inhibition in patients with non-ST-segment elevation acute coronary syndrome (EARLY ACS) trial. Circulation. 2011;123:722–730. - PubMed
-
- Olivier C.B., Diehl P., Schnabel K., Weik P., Zhou Q., Bode C., Moser M. Third generation P2Y12 antagonists inhibit platelet aggregation more effectively than clopidogrel in a myocardial infarction registry. Thromb. Haemost. 2014;111:266–272. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

