Lipid emulsions for parenterally fed preterm infants
- PMID: 31158919
- PMCID: PMC6953211
- DOI: 10.1002/14651858.CD013163.pub2
Lipid emulsions for parenterally fed preterm infants
Abstract
Background: Conventionally used soybean oil-based lipid emulsion (S-LE) have high polyunsaturated fatty acid (PUFA) content and phytosterols that may contribute to adverse effects in preterm infants. The newer lipid emulsions (LE) from different lipid sources are currently available for use in preterm infants.
Objectives: To compare the safety and efficacy of all LE for parenteral nutrition (PN) in preterm infants (less than 37 weeks' gestation) including preterm infants with surgical conditions or parenteral nutrition-associated liver disease (PNALD)/cholestasis using direct comparisons and pair-wise meta-analyses.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 5), MEDLINE (1946 to 18 June 2018), Embase (1974 to 18 July 2018), CINAHL (1982 to 18 June 2018), MIDRIS (1971 to 31 May 2018), conference proceedings, trial registries (ClinicalTrials.gov and WHO's Trials Registry and Platform), and reference lists of retrieved articles.
Selection criteria: Randomised or quasi-randomised controlled studies in preterm infants with or without surgical conditions or PNALD within the first six months of life.
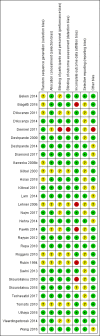
Data collection and analysis: Data collection and analysis conformed to the methods of Cochrane Neonatal. We used the GRADE approach to assess the quality of evidence for important outcomes in addition to reporting statistical significance of results.
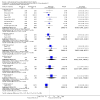
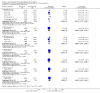
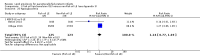
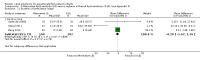
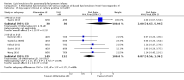
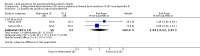
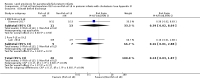
Main results: We included 29 studies (n = 2037) in this review. LE were classified in three broad groups: 1. all fish oil-containing LE including pure fish oil-LE (F-LE) and multisource LE (e.g. medium-chain triglycerides (MCT)-olive-fish-soybean oil-LE (MOFS-LE), MCT-fish-soybean oil-LE (MFS-LE) and olive-fish-soybean oil-LE (OFS-LE); 2. conventional S-LE; 3. alternative-LE (e.g. MCT-soybean oil-LE (MS-LE), olive-soybean oil-LE and borage oil-based LE).We considered the following broad comparisons: fish oil LE versus non-fish oil LE; fish oil LE versus another fish oil LE; alternative-LE versus S-LE; alternative-LE versus another alternative-LE in preterm infants less than 37 weeks' gestation, preterm infants with surgical conditions and preterm infants with PNALD/cholestasis. Separate subgroup comparisons of each LE preparation were included within these broader groups.Most studies in preterm infants used PN for mean duration of four weeks or less and for longer duration in infants with cholestasis or surgical conditions.We defined the primary outcome of PNALD/cholestasis as conjugated bilirubin (Cbil) 2 mg/dL or greater and resolution of PNALD/cholestasis as Cbil less than 2 mg/dL. There was heterogeneity in definitions used by the included studies with Cbil cut-offs ranging from 17.1 μmol/L (1 mg/dL) up to 50 μmol/L (about 3 mg/dL).In preterm infants, meta-analysis found no evidence of a difference in the incidence of PNALD/cholestasis (Cbil cut-off: 2 mg/dl) between fish oil-LEs and all non-fish oil LEs (typical risk ratio (RR) 0.61, 95% confidence interval (CI) 0.24 to 1.56; typical risk difference (RD) -0.03, 95% CI -0.08 to 0.02; 4 studies; n = 328; low-quality evidence).We also considered an outcome allowing for any definition of PNALD (different Cbil cutoffs). In the meta-analysis for PNALD/cholestasis, using any definition and restricted to low or unclear risk of bias studies, there was no evidence of a difference between fish oil LE and all non-fish oil LE for incidence of cholestasis (typical RR 0.80, 95% CI 0.53 to 1.21; typical RD -0.02, 95% CI -0.05 to 0.02; 10 studies; n = 1024; low-quality evidence). There was no evidence of difference in subgroup meta-analyses of individual LE types in any comparison.In preterm infants with surgical conditions or cholestasis, there was only one small study each reporting no evidence of a difference in incidence or resolution of cholestasis respectively with use of a pure F-LE versus S-LE (using a Cbil cut-off of 2 mg/dL).In preterm infants with PNALD/cholestasis (using any definition), the meta-analysis showed significantly less cholestasis with the use of fish oil-LE compared to S-LE (typical RR 0.54, 95% CI 0.32 to 0.91; typical RD -0.39, 95% CI -0.65 to -0.12; number needed to treat for an additional beneficial outcome (NNTB) 3, 95% CI 2 to 9; 2 studies; n = 40; very low-quality evidence). However, this outcome had a very low number of participants from two small studies with methodological differences, one of which was terminated early, increasing the uncertainty about effect estimates.There were no differences between LE types in pair-wise meta-analyses for growth in preterm infants. There was paucity of studies in preterm infants with surgical conditions or cholestasis to perform meta-analyses for growth and most other outcomes.In the secondary outcomes for preterm infants, there was no difference between fish-oil LE and non-fish oil LE in meta-analysis for severe retinopathy of prematurity (ROP) (stage 3 or greater, or requiring surgery: typical RR 0.80, 95% CI 0.55 to 1.16; typical RD -0.03, 95% CI -0.07 to 0.02; 7 studies; n = 731; very low-quality evidence). There were no differences in the LE types in pair-wise meta-analyses for death, bronchopulmonary dysplasia (BPD), ventilation duration, patent ductus arteriosus, sepsis, necrotising enterocolitis, intraventricular haemorrhage, periventricular leukomalacia, jaundice, hyperglycaemia, hypertriglyceridaemia, intrahepatocellular lipid content and conjugated bilirubin levels in any comparison.In surgical infants, one study (n = 19) reported no differences in death, sepsis rates, Cbil and neurodevelopmental outcomes with pure F-LE versus S-LE.In infants with cholestasis, there were no evidence of differences in death or sepsis in meta-analyses between fish oil-LE and S-LE; (2 studies; n = 40; very low-quality evidence).
Authors' conclusions: In the current review, we did not find any particular LE with or without fish oil to be better than another LE in preterm infants for prevention of PNALD/cholestasis, growth, mortality, ROP, BPD and other neonatal outcomes.In preterm infants with surgical conditions or cholestasis, there is currently insufficient evidence from randomised studies to determine with any certainty if fish oil LEs offer advantage in prevention or resolution of cholestasis or in any other clinical outcome.Further research, with larger well-designed trials, is warranted to evaluate the ideal composition of LE in preterm infants and the role of fish oil-containing and other LEs in the prevention and resolution of PNALD, ROP and other clinical outcomes.
Conflict of interest statement
VK: none.
MM: none.
RS: none.
Figures










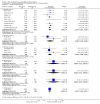



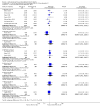
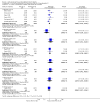




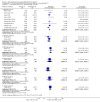




































































Update of
- doi: 10.1002/14651858.CD013163
Similar articles
-
Alternative lipid emulsions versus pure soy oil based lipid emulsions for parenterally fed preterm infants.Cochrane Database Syst Rev. 2015 Dec 2;2015(12):CD009172. doi: 10.1002/14651858.CD009172.pub2. Cochrane Database Syst Rev. 2015. PMID: 26630252 Free PMC article. Review.
-
Lipid emulsions for parenterally fed term and late preterm infants.Cochrane Database Syst Rev. 2019 Jun 4;6(6):CD013171. doi: 10.1002/14651858.CD013171.pub2. Cochrane Database Syst Rev. 2019. PMID: 31158920 Free PMC article.
-
Inositol in preterm infants at risk for or having respiratory distress syndrome.Cochrane Database Syst Rev. 2019 Jul 8;7(7):CD000366. doi: 10.1002/14651858.CD000366.pub4. Cochrane Database Syst Rev. 2019. PMID: 31283839 Free PMC article.
-
Higher versus lower amino acid intake in parenteral nutrition for newborn infants.Cochrane Database Syst Rev. 2018 Mar 5;3(3):CD005949. doi: 10.1002/14651858.CD005949.pub2. Cochrane Database Syst Rev. 2018. PMID: 29505664 Free PMC article. Review.
-
Early erythropoiesis-stimulating agents in preterm or low birth weight infants.Cochrane Database Syst Rev. 2020 Feb 11;2(2):CD004863. doi: 10.1002/14651858.CD004863.pub6. Cochrane Database Syst Rev. 2020. PMID: 32048730 Free PMC article.
Cited by
-
Risk of Liver Injury Associated with Intravenous Lipid Emulsions: A Prescription Sequence Symmetry Analysis.Front Pharmacol. 2021 Mar 1;12:589091. doi: 10.3389/fphar.2021.589091. eCollection 2021. Front Pharmacol. 2021. PMID: 33732151 Free PMC article.
-
Alternative lipid emulsions versus pure soy oil based lipid emulsions for parenterally fed preterm infants.Cochrane Database Syst Rev. 2015 Dec 2;2015(12):CD009172. doi: 10.1002/14651858.CD009172.pub2. Cochrane Database Syst Rev. 2015. PMID: 26630252 Free PMC article. Review.
-
Impact of Parenteral Lipid Emulsion Components on Cholestatic Liver Disease in Neonates.Nutrients. 2021 Feb 4;13(2):508. doi: 10.3390/nu13020508. Nutrients. 2021. PMID: 33557154 Free PMC article. Review.
-
The efficacy and safety of peripheral intravenous parenteral nutrition vs 10% glucose in preterm infants born 30 to 33 weeks' gestation: a randomised controlled trial.BMC Pediatr. 2020 Aug 17;20(1):384. doi: 10.1186/s12887-020-02280-w. BMC Pediatr. 2020. PMID: 32799841 Free PMC article. Clinical Trial.
-
IFALD in children: What's new? A narrative review.Front Nutr. 2022 Jul 25;9:928371. doi: 10.3389/fnut.2022.928371. eCollection 2022. Front Nutr. 2022. PMID: 35958249 Free PMC article. Review.
References
References to studies included in this review
Beken 2014 {published data only}
-
- Beken S, Dilli D, Fettah ND, Kabatas EU, Zenciroqlu A, Okumus N. The influence of fish‐oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomised controlled trial. Early Human Development 2014;90(1):27‐31. [DOI: 10.1016/j.earlhumdev.2013.11.002; PUBMED: 24314586] - DOI - PubMed
Biagetti 2016 {published data only}
-
- Biagetti C, Vedovelli L, Savini, S, Simonato M, D'Ascenzo R, Pompilio A, et al. Double blind exploratory study on de novo lipogenesis in preterm infants on parenteral nutrition with a lipid emulsion containing 10% fish oil. Clinical Nutrition 2016;35(2):337‐43. [DOI: 10.1016/j.clnu.2015.04.005; PUBMED: 25912232] - DOI - PubMed
D'Ascenzo 2011 {published data only}
-
- D'Ascenzo R, D'Egidio S, Angelini L, Bellagamba MP, Manna M, Pompilio A, et al. Parenteral nutrition of preterm infants with a lipid emulsion containing 10% fish oil: effect on plasma lipids and long‐chain polyunsaturated fatty acids. Journal of Pediatrics 2011;159(1):33‐8.e1. [DOI: 10.1016/j.jpeds.2010.12.052; PUBMED: 21362575] - DOI - PubMed
D'Ascenzo 2014 {published data only}
-
- D'Ascenzo R, Savini S, Biagetti C, Bellagamba MP, Marchionni P, Pompilio A, et al. Higher docosahexaenoic acid, lower arachidonic acid and reduced lipid tolerance with high doses of a lipid emulsion containing 15% fish oil: a randomised clinical trial. Clinical Nutrition 2014;33(6):1002‐9. - PubMed
Demirel 2011 {published data only (unpublished sought but not used)}
-
- Demirel G, Oguz SS, Celik IH, Erdeve O, Uras N, Dilmen U. The metabolic effects of two different lipid emulsions used in parenterally fed premature infants – a randomised comparative study. Early Human Development 2012;88(7):499‐501. [DOI: 10.1016/j.earlhumdev.2011.12.008; PUBMED: 22245235] - DOI - PubMed
Deshpande 2009 {published data only (unpublished sought but not used)}
-
- Deshpande GC, Simmer K, Mori T, Croft K. Parenteral lipid emulsions based on olive oil compared with soybean oil in preterm (≤ 28 weeks' gestation) neonates: a randomised controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2009;49(5):619‐25. [DOI: 10.1097/MPG.0b013e31819ca1b8; PUBMED: 19644398] - DOI - PubMed
Deshpande 2014 {published data only}
Diamond 2017 {published data only}
-
- Diamond IR, Grant RC, Pencharz PB, Silva N, Feldman BM, Fitzgerald P, et al. Preventing the progression of intestinal failure‐associated liver disease in infants using a composite lipid emulsion: a pilot randomized controlled trial of SMOFlipid. JPEN. Journal of Parenteral and Enteral Nutrition 2017; Vol. 41, issue 5:866‐77. [DOI: 10.1177/0148607115626921; PUBMED: 26838529] - DOI - PubMed
Gawecka 2008b {published data only (unpublished sought but not used)}
-
- Gawecka A, Michalkiewicz J, Kornacka MK, Luckiewicz B, Kubiszewska I. Immunologic properties differ in preterm infants fed olive oil vs soy‐based lipid emulsions during parenteral nutrition. JPEN. Journal of Parenteral and Enteral Nutrition 2008;32(4):448‐53. [DOI: 10.1177/0148607108319802; PUBMED: 18596318] - DOI - PubMed
Göbel 2003 {published data only}
-
- Göbel Y, Koletzko B, Böhles HJ, Engelsberger I, Forget D, Brun A, et al. Parenteral fat emulsions based on olive and soybean oils: a randomised clinical trial in preterm infants. Journal of Pediatric Gastroenterology and Nutrition 2003;37(2):161‐7. [PUBMED: 12883303] - PubMed
Hsiao 2018 {published data only}
-
- Hsiao CC, Lin HC, Chang YJ, Yang SP, Tsao LY, Lee CH, et al. Intravenous fish oil containing lipid emulsion attenuates inflammatory cytokines and the development of bronchopulmonary dysplasia in very premature infants: a double‐blind, randomised controlled trial. Clinical Nutrition 2018, (18):31148‐8. [DOI: 10.1016/j.clnu.2018.06.929; PUBMED: 29941233] - DOI - PubMed
Köksal 2011 {published and unpublished data}
Lam 2014 {published data only}
-
- Lam HS, Tam YH, Poon TC, Cheung HM, Yu X, Chan BP, et al. A double‐blind randomised controlled trial of fish oil‐based versus soy‐based lipid preparations in the treatment of infants with parenteral nutrition‐associated cholestasis. Neonatology 2014;4(105):290‐6. [DOI: 10.1159/000358267; PUBMED: 24576844] - DOI - PubMed
Lehner 2006 {published data only (unpublished sought but not used)}
Najm 2017 {published data only}
-
- Najm S, Löfqvist C, Hellgren G, Engström E, Lundgrenb P, Hård AL, et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: a randomised controlled trial. Clinical Nutrition ESPEN 2017;20:17‐23. [DOI: 10.1016/j.clnesp.2017.04.004; PUBMED: 29072164] - DOI - PMC - PubMed
Nehra 2014 {published data only}
-
- Nehra D, Fallon EM, Potemkin AK, Voss SD, Mitchell PD, Valim C, et al. A comparison of 2 intravenous lipid emulsions: interim analysis of a randomised controlled trial. JPEN. Journal of Parenteral and Enteral Nutrition 2014;38(6):693‐701. [DOI: 10.1177/0148607113492549; PUBMED: 23770843] - DOI - PMC - PubMed
Pawlik 2014 {published data only}
-
- Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP. Fish‐oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: a prospective, randomised study. JPEN. Journal of Parenteral and Enteral Nutrition 2014;38(6):711‐6. [DOI: 10.1177/0148607113499373; PUBMED: 23963690] - DOI - PubMed
Rayyan 2012 {published data only (unpublished sought but not used)}
-
- Rayyan M, Devlieger H, Jochum F, Allegaert K. Short‐term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium‐chain triglycerides, and fish oil: a randomised double‐blind study in preterm infants. Journal of Parenteral and Enteral Nutrition 2012;36(1 Suppl):81S‐94S. [DOI: 10.1177/0148607111424411; PUBMED: 22237883] - DOI - PMC - PubMed
Repa 2018 {published data only}
-
- Repa A, Binder C, Thanhaeuser M, Kreissl A, Pablik E, Huber‐Dangl M, et al. A mixed lipid emulsion for prevention of parenteral nutrition associated cholestasis in extremely low birth weight infants: a randomized clinical trial. Journal of Pediatrics 2018;194:87‐93. [DOI: 10.1016/j.jpeds.2017.11.012; PUBMED: 29269199] - DOI - PMC - PubMed
Roggero 2010 {published data only}
Rubin 1994 {published data only}
-
- Rubin M, Moser A, Naor N, Merlob P, Pakula R, Sirota L. Effect of three intravenously administered fat emulsions containing different concentrations of fatty acids on the plasma fatty acid composition of premature infants. Journal of Pediatrics 1994;125(4):596‐602. [PUBMED: 7931881] - PubMed
Savini 2013 {published data only (unpublished sought but not used)}
-
- Savini S, D'Ascenzo R, Biagetti C, Serpentini G, Pompilio A, Bartoli A, et al. The effect of 5 intravenous lipid emulsions on plasma phytosterols in preterm infants receiving parenteral nutrition: a randomised clinical trial. American Journal of Clinical Nutrition 2013;98(2):312‐8. [DOI: 10.3945/ajcn.112.056556; PUBMED: 23761482] - DOI - PubMed
Skouroliakou 2010 {published data only (unpublished sought but not used)}
-
- Skouroliakou M, Konstantinou D, Koutri K, Kakavelaki C, Stathopoulou M, Antoniadi M, et al. A double‐blind, randomised clinical trial of the effect of omega‐3 fatty acids on the oxidative stress of preterm neonates fed through parenteral nutrition. European Journal of Clinical Nutrition 2010;64(9):940‐7. [DOI: 10.1038/ejcn.2010.98; PUBMED: 20551967] - DOI - PubMed
Skouroliakou 2016 {published data only}
-
- Skouroliakou M, Konstantinou D, Agakidis C, Kaliora A, Kalogeropoulos N, Massara P, et al. Parenteral MCT/omega‐3 polyunsaturated fatty acid‐enriched intravenous fat emulsion is associated with cytokine and fatty acid profiles consistent with attenuated inflammatory response in preterm neonates: a randomized, double‐blind clinical trial. Nutrition in Clinical Practice 2016;31(2):235‐44. - PubMed
Techasatid 2017 {published data only}
-
- Techasatid W, Sapsaprang S, Tantiyavarong P, Luvira A. Effectiveness of multicomponent lipid emulsion in preterm infants requiring parenteral nutrition: a two‐center, double‐blind randomized clinical trial. Journal of the Medical Association of Thai 2017;100(9):972‐9.
Tomsits 2010 {published data only (unpublished sought but not used)}
-
- Tomsits E, Pataki M, Tölgyesi A, Fekete G, Rischak K, Szollár L. Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium‐chain triglycerides, olive oil, and fish oil: a randomised, double‐blind clinical trial in premature infants requiring parenteral nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;51(4):514‐21. [DOI: 10.1097/MPG.0b013e3181de210c; PUBMED: 20531018] - DOI - PubMed
Uthaya 2016 {published data only}
-
- Uthaya S, Liu X, Babalis D, Dore C, Warwick J, Bell J, et al. Nutritional evaluation and optimisation in neonates: a randomised, double‐blind controlled trial of amino acid regimen and intravenous lipid composition in preterm parenteral nutrition. American Journal of Clinical Nutrition 2016;103:1443‐52. [PUBMED: 27030860] - PMC - PubMed
Vlaardingerbroek 2014 {published data only}
-
- Vlaardingerbroek H, Vermeulen MJ, Carnielli VP, Vaz FM, Akker CH, Goudoever JB. Growth and fatty acid profiles of VLBW infants receiving a multicomponent lipid emulsion from birth. Journal of Pediatric Gastroenterology and Nutrition 2014;58(4):417‐27. [DOI: 10.1097/MPG.0000000000000280; PUBMED: 24667866] - DOI - PubMed
-
- Vlaardingerbroek H, Vermeulen MJ, Rook D, Akker CH, Dorst K, Wattimena JL, et al. Safety and efficacy of early parenteral lipid and high‐dose amino acid administration to very low birth weight infants. The Journal of Pediatrics 2013;163(3):638‐44.e1‐5. [DOI: 10.1016/j.jpeds.2013.03.059] - DOI - PubMed
Wang 2016 {published data only}
-
- Wang Y, Zhou KJ, Tang QY, Hong L, Feng Y, Lu LN, et al. Effect of an olive oil‐based lipid emulsion compared with a soybean oil‐based lipid emulsion on liver chemistry and bile acid composition in preterm infants receiving parenteral nutrition: a double‐blind, randomized trial. Journal of Parenteral and Enteral Nutrition 2016;40(6):842‐50. [DOI: 10.1177/0148607114566853; PUBMED: 25560678] - DOI - PubMed
References to studies excluded from this review
Angsten 2002 {published data only}
-
- Angsten G, Boberg M, Cederblad G, Meurling S, Stiernström H. Metabolic effects in neonates receiving intravenous medium‐chain triglycerides. Acta Paediatrica 2002;91(2):188‐97. [PUBMED: 11952008] - PubMed
Ariyawangso 2014 {published data only}
-
- Ariyawangso U, Puttilerpong C, Ratanachuek S, Anuntkosol M. Short‐term safety and efficacy of fish‐oil emulsions on the prevention of parenteral nutrition‐associated liver disease in surgical neonates: a randomised controlled trial. Thai Journal of Pharmaceutical Sciences 2014;38(4):202‐9.
Lima 1988 {published data only}
-
- Lima LA, Murphy JF, Stansbie D, Rowlandson P, Gray OP. Neonatal parenteral nutrition with a fat emulsion containing medium chain triglycerides. Acta Paediatrica Scandinavica 1988;77(3):332‐9. [PUBMED: 3133924] - PubMed
Magnusson 1997 {published data only}
-
- Magnusson G, Boberg M, Cederblad G, Meurling S. Plasma and tissue levels of lipids, fatty acids and plasma carnitine in neonates receiving a new fat emulsion. Acta Paediatrica 1997;86(6):638‐44. [PUBMED: 9202801] - PubMed
Webb 2008 {published data only}
Wilson 1997 {published data only}
References to studies awaiting assessment
Karagiozoglou‐Lampoudi 2012 {published data only (unpublished sought but not used)}
-
- Karagiozoglou‐Lampoudi T, Skouroliakou M, Konstantinou D, Agakidis C, Delikou N, Koutri K, et al. Omega‐3‐polyunsaturated fatty acid – enriched parenteral lipid emulsion and prevention of cholestasis in preterm infants. Comparison with soybean‐based lipid emulsion. European Journal of Hospital Pharmacy: Science and Practice 2012;19(2):221‐2. [DOI: ]
NCT03275090 {unpublished data only}
-
- Wahba Y, Abdelkareem M, Shouman B, Mesbah A. The effects of two different intravenous lipid emulsions on the outcomes of preterm infants with sepsis: a randomised controlled trial. Journal of Pediatric Care 2018;4. [DOI: 10.21767/2471-805X-C2-009] - DOI
Wang 2016b {published data only}
-
- Wang Y, Feng Y, Lu LN, Wang WP, He ZJ, Xie LJ, et al. The effects of different lipid emulsions on the lipid profile, fatty acid composition, and antioxidant capacity of preterm infants: a double‐blind, randomised clinical trial. Clinical Nutrition 2016;35(5):1023‐31. [DOI: 10.1016/j.clnu.2015.10.011; PUBMED: 26561301] - DOI - PubMed
Additional references
AAP 1985
-
- American Academy of Pediatrics Committee on Nutrition. Nutritional needs of low‐birth‐weight infants. Pediatrics 1985;75(5):976‐86. [PUBMED: 3921937] - PubMed
Ahmad 2010
Beauchamp 2005
Bell 1978
Buenestado 2006
-
- Buenestado A, Cortijo J, Sanz MJ, Naim‐Abu‐Nabah Y, Martinez‐Losa M, Mata M, et al. Olive oil‐based lipid emulsion's neutral effects on neutrophil functions and leukocyte–endothelial cell interactions. Journal of Parenteral and Enteral Nutrition 2006;30(4):286‐96. [DOI: 10.1177/0148607106030004286; PUBMED: 16804125] - DOI - PubMed
Christensen 2007
Cober 2010
de Meijer 2009
de Vries 1992
-
- Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behavioural Brain Research 1992;49(1):1‐6. [PUBMED: 1388792] - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG, editor(s), Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2017.
Driscoll 2008
Ehrenkranz 2000
-
- Ehrenkranz RA. Growth outcomes of very low‐birth weight infants in the newborn intensive care unit. Clinics in Perinatology 2000;27(2):325‐45. [PUBMED: 10863653] - PubMed
Fenton 2017
Furukawa 2006
Fürst 2000
Gawecka 2008a
-
- Gawecka A, Kornacka MK, Luckiewicz B, Rudzinska I. Tolerance of two lipid emulsions used in parenterally‐fed premature infants – a comparative study. Medycyna Wieku Rozwojowego 2008;12(3):782‐8. [PUBMED: 19305031] - PubMed
Gawecka 2008c
-
- Gawecka A, Michalkiewicz J, Kornacka MK, Luckiewicz B, Kubiszewska I. Immunologic properties differ in preterm infants fed olive oil vs soy‐based lipid emulsions during parenteral nutrition. Journal of Parenteral and Enteral Nutrition 2008;32(4):448‐53. [DOI: 10.1177/0148607108319802; PUBMED: 18596318] - DOI - PubMed
Gitto 2001
-
- Gitto E, Karbownik M, Reiter RJ, Tan DX, Cuzzocrea S, Chiurazzi P, et al. Effects of melatonin treatment in septic newborns. Pediatric Research 2001;50(6):756‐60. [PUBMED: 11726736] - PubMed
Gogos 1995
-
- Gogos CA, Kalfarentzos F. Total parenteral nutrition and immune system activity: a review. Nutrition 1995;11(4):339‐44. [PUBMED: 8580573] - PubMed
Goulet 1999
-
- Goulet O, Potter S, Antebi H, Driss F, Colomb V, Bereziat G, et al. Long‐term efficacy and safety of a new olive oil‐based intravenous fat emulsion in pediatric patients: a double‐blind randomised study. American Journal of Clinical Nutrition 1999;70(3):338‐45. [DOI: 10.1093/ajcn/70.3.338; PUBMED: 10479195] - DOI - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 22 January 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Granato 2000
Grimm 2005
-
- Grimm H. A balanced lipid emulsion – a new concept in parenteral nutrition. Clinical Nutrition Supplements 2005;1(3):25‐30. [DOI: ]
Gura 2005
-
- Gura KM, Parsons SK, Bechard LJ, Henderson T, Dorsey M, Phipatanakul W, et al. Use of a fish oil‐based lipid emulsion to treat essential fatty acid deficiency in a soy allergic patient receiving parenteral nutrition. Clinical Nutrition 2005;24(5):839‐47. [DOI: 10.1016/j.clnu.2005.05.020; PUBMED: 16029913] - DOI - PubMed
Hay 2008
Heird 2005
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook.
Hojsak 2016
-
- Hojsak I, Colomb V, Braegger C, Bronsky J, Campoy C, Domellof M, et al. ESPGHAN Committee on Nutrition Position Paper. Intravenous lipid emulsions and risk of hepatotoxicity in infants and children: a systematic review and meta‐analysis. Journal of Pediatric Gastroenterology and Nutrition 2016;62(5):776‐92. [PUBMED: 26825766] - PubMed
Hozo 2005
ICROP 2005
Koletzko 2001
-
- Koletzko B, Agostoni C, Carlson SE, Clandinin T, Hornstra G, Neuringer M, et al. Long chain polyunsaturated fatty acids (LC‐PUFA) and perinatal development. Acta Paediatrica 2001;90(4):460‐4. [PUBMED: 11332943] - PubMed
Koletzko 2005
-
- Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). Journal of Pediatric Gastroenterology and Nutrition 2005;41 Suppl 2:1‐87. [PUBMED: 16254497] - PubMed
Kotiya 2016
Krohn 2006
-
- Krohn K, Koletzko B. Parenteral lipid emulsions in paediatrics. Current Opinion in Clinical Nutrition and Metabolic Care 2006;9(3):319‐23. [DOI: 10.1097/01.mco.0000222118.76536.ad; PUBMED: 16607135] - DOI - PubMed
Lam 2014
-
- Lam HS, Tam YH, Poon TC, Cheung HM, Yu X, Chan BP, et al. A double‐blind randomised controlled trial of fish oil‐based versus soy‐based lipid preparations in the treatment of infants with parenteral nutrition‐associated cholestasis. Neonatology 2014;105(4):290‐6. [DOI: 10.1159/000358267; PUBMED: 24576844] - DOI - PubMed
Lapillonne 2013
Lee 1993
-
- Lee EJ, Simmer K, Gibson, RA. Essential fatty acid deficiency in parenterally fed preterm infants. Journal of Paediatrics and Child Health 1993;29(1):51‐5. [PUBMED: 8461181] - PubMed
Lekka 2004
McNaught 1997
-
- McNaught AD, Wilkinson A. IUPAC. Compendium of Chemical Terminology (the "Gold Book"). 2nd Edition. Oxford: Blackwell Scientific Publications, 1997.
Moher 2009
Mylonas 1999
-
- Mylonas C, Kouretas D. Lipid peroxidation and tissue damage. In Vivo 1999;13(3):295‐309. [PUBMED: 10459507] - PubMed
Palmblad 1991
-
- Palmblad J. Intravenous lipid emulsions and host defense – a critical review. Clinical Nutrition 1991;10(6):303‐8. [DOI: ; PUBMED: 16839936] - PubMed
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] - PubMed
Park 2015
Pawlik 2011
Pereira‐da‐Silva 2017
-
- Pereira‐da‐Silva L, Nobrega S, Rosa ML, Alves M, Pita A, Virella D, et al. Parenteral nutrition‐associated cholestasis and triglyceridemia in surgical term and near‐term neonates: a pilot randomized controlled trial of two mixed intravenous lipid emulsions. Clinical Nutrition ESPEN 2017;22:7‐12. [PUBMED: 29415837] - PubMed
Pitkanen 1991
Puder 2009
Putet 2000
-
- Putet G. Lipid metabolism of the micropremie. Clinics in Perinatology 2000;27(1):57‐69. [PUBMED: 10690564] - PubMed
Reimund 2004
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2008
Roggero 2007
-
- Roggero P, Giannì ML, Amato O, Agosti M, Fumagalli M, Mosca F. Measuring the body composition of preterm and term neonates: from research to clinical applications. Journal of Pediatric Gastroenterology and Nutrition 2007;45 Suppl 3:S159–62. Erratum in: Journal of Pediatric Gastroenterology and Nutrition. 2009;48(1):121‐2. [DOI: 10.1097/01.mpg.0000302964.85922.1a; PUBMED: 18185084] - DOI - PubMed
Rubin 1995
-
- Rubin M, Naor N, Sirota L, Moser A, Pakula R, Harell D, et al. Are bilirubin and plasma lipid profiles of premature infants dependent on the lipid emulsion infused?. Journal of Pediatric Gastroenterology and Nutrition 1995;21(1):25‐30. [PUBMED: 8576810] - PubMed
Sala‐Vila 2007
SanGiovanni 2000
-
- SanGiovanni JP, Berkey CS, Dwyer JT, Colditz GA. Dietary essential fatty acids, long‐chain polyunsaturated fatty acids, and visual resolution acuity in healthy full term infants: a systematic review. Early Human Development 2000;57(3):165‐88. [PUBMED: 10742608] - PubMed
Schock 2001
-
- Schock BC, Sweet DG, Halliday HL, Young IS, Ennis M. Oxidative stress in lavage fluid of preterm infants at risk of chronic lung disease. American Journal of Physiology. Lung Cellular and Molecular Physiology 2001;281(6):L1386‐91. [DOI: 10.1152/ajplung.2001.281.6.L1386; PUBMED: 11704534] - DOI - PubMed
Scholtens 2009
-
- Scholtens S, Wijga AH, Smit HA, Brunekreef B, Jongste JC, Gerritsen J, et al. Long‐chain polyunsaturated fatty acids in breast milk and early weight gain in breast‐fed infants. British Journal of Nutrition 2009;101(1):116‐21. [PUBMED: 18492299] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sinclair 2011
Skouroliakou 2012
-
- Skouroliakou M, Konstantinou D, Agakidis C, Delikou N, Koutri K, Antoniadi M, et al. Cholestasis, bronchopulmonary dysplasia, and lipid profile in preterm infants receiving MCT/ω‐3‐PUFA‐containing or soybean‐based lipid emulsions. Nutrition in Clinical Practice 2012;27(6):817‐24. [DOI: 10.1177/0884533612454547; PUBMED: 22878361] - DOI - PubMed
Sterne 2017
-
- Sterne JA, Egger M, Moher D, Boutron I. Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Stoll 2002
-
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late‐onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 PT 1):285‐91. [PUBMED: 12165580] - PubMed
Van Kempen 2006
-
- Kempen AA, Crabben SN, Ackermans MT, Endert E, Kok JH, Sauerwein HP. Stimulation of gluconeogenesis by intravenous lipids in preterm infants: response depends on fatty acid profile. American Journal of Physiology. Endocrinology and Metabolism 2006;290(4):E723‐30. [DOI: 10.1152/ajpendo.00303.2005; PUBMED: 16291574] - DOI - PubMed
Vanek 2012
Vayalthrikkovil 2017
-
- Vayalthrikkovil S, Bashir RA, Rabi Y, Amin H, Spence JM, Robertson HL, et al. Parenteral fish‐oil lipid emulsions in the prevention of severe retinopathy of prematurity: a systematic review and meta‐analysis. American Journal of Perinatology 2017;34(7):705‐15. [DOI: 10.1055/s-0036-1597131; PUBMED: 27992937] - DOI - PubMed
Vlaardingerbroek 2012
-
- Vlaardingerbroek H, Veldhorst M, Spronk S, Akker CH, Goudoever JB. Parenteral lipid administration to very‐low‐birth‐weight infants – early introduction of lipids and use of new lipid emulsions: a systematic review and meta‐analysis. American Journal of Clinical Nutrition 2012;96(2):255‐68. [DOI: 10.3945/ajcn.112.040717; PUBMED: 22743312] - DOI - PubMed
Waitzberg 2006
Walsh 2004
-
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305‐11. [PUBMED: 15520112] - PubMed
Wanten 2007
Xu 2012
-
- Xu ZW, Li YS. Pathogenesis and treatment of parenteral nutrition‐associated liver disease. Hepatobiliary & Pancreatic Diseases International 2012;11(6):586‐93. [PUBMED: 23232629] - PubMed
References to other published versions of this review
Kapoor 2015
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

