A PRDM16-Driven Metabolic Signal from Adipocytes Regulates Precursor Cell Fate
- PMID: 31155495
- PMCID: PMC6836679
- DOI: 10.1016/j.cmet.2019.05.005
A PRDM16-Driven Metabolic Signal from Adipocytes Regulates Precursor Cell Fate
Abstract
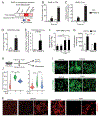
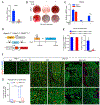
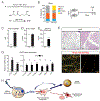
The precursor cells for metabolically beneficial beige adipocytes can alternatively become fibrogenic and contribute to adipose fibrosis. We found that cold exposure or β3-adrenergic agonist treatment of mice decreased the fibrogenic profile of precursor cells and stimulated beige adipocyte differentiation. This fibrogenic-to-adipogenic transition was impaired in aged animals, correlating with reduced adipocyte expression of the transcription factor PRDM16. Genetic loss of Prdm16 mimicked the effect of aging in promoting fibrosis, whereas increasing PRDM16 in aged mice decreased fibrosis and restored beige adipose development. PRDM16-expressing adipose cells secreted the metabolite β-hydroxybutyrate (BHB), which blocked precursor fibrogenesis and facilitated beige adipogenesis. BHB catabolism in precursor cells, mediated by BDH1, was required for beige fat differentiation in vivo. Finally, dietary BHB supplementation in aged animals reduced adipose fibrosis and promoted beige fat formation. Together, our results demonstrate that adipocytes secrete a metabolite signal that controls beige fat remodeling.
Keywords: BDH1; PRDM16; UCP1; adipose fibrosis; beige fat; beta hydroxybutyrate; brown fat; fibro-adipogenic progenitor.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures
Similar articles
-
Resveratrol derivative BTM-0512 mitigates obesity by promoting beige remodeling of subcutaneous preadipocytes.Acta Biochim Biophys Sin (Shanghai). 2017 Apr 1;49(4):318-327. doi: 10.1093/abbs/gmx009. Acta Biochim Biophys Sin (Shanghai). 2017. PMID: 28338809
-
MiR-27 orchestrates the transcriptional regulation of brown adipogenesis.Metabolism. 2014 Feb;63(2):272-82. doi: 10.1016/j.metabol.2013.10.004. Epub 2013 Oct 24. Metabolism. 2014. PMID: 24238035
-
Three-Dimensional Adipose Tissue Imaging Reveals Regional Variation in Beige Fat Biogenesis and PRDM16-Dependent Sympathetic Neurite Density.Cell Metab. 2018 Jan 9;27(1):226-236.e3. doi: 10.1016/j.cmet.2017.12.011. Cell Metab. 2018. PMID: 29320703
-
Role of PRDM16 in the activation of brown fat programming. Relevance to the development of obesity.Histol Histopathol. 2013 Nov;28(11):1411-25. doi: 10.14670/HH-28.1411. Epub 2013 Jun 17. Histol Histopathol. 2013. PMID: 23771475 Review.
-
[Evaluation of Naturally Occurring Compounds Regulating Brown/Beige Adipocyte Differentiation].Yakugaku Zasshi. 2019;139(6):861-866. doi: 10.1248/yakushi.18-00173-3. Yakugaku Zasshi. 2019. PMID: 31155526 Review. Japanese.
Cited by
-
ACSS3 in brown fat drives propionate catabolism and its deficiency leads to autophagy and systemic metabolic dysfunction.Clin Transl Med. 2022 Feb;12(2):e665. doi: 10.1002/ctm2.665. Clin Transl Med. 2022. PMID: 35184387 Free PMC article.
-
Wireless optogenetics protects against obesity via stimulation of non-canonical fat thermogenesis.Nat Commun. 2020 Apr 7;11(1):1730. doi: 10.1038/s41467-020-15589-y. Nat Commun. 2020. PMID: 32265443 Free PMC article.
-
Exercise training remodels inguinal white adipose tissue through adaptations in innervation, vascularization, and the extracellular matrix.Cell Rep. 2023 Apr 25;42(4):112392. doi: 10.1016/j.celrep.2023.112392. Epub 2023 Apr 13. Cell Rep. 2023. PMID: 37058410 Free PMC article.
-
The multifaceted progenitor fates in healthy or unhealthy adipose tissue during obesity.Rev Endocr Metab Disord. 2021 Dec;22(4):1111-1119. doi: 10.1007/s11154-021-09662-0. Epub 2021 Jun 8. Rev Endocr Metab Disord. 2021. PMID: 34105090 Review.
-
Inside the Biology of the β3-Adrenoceptor.Biomolecules. 2024 Jan 29;14(2):159. doi: 10.3390/biom14020159. Biomolecules. 2024. PMID: 38397396 Free PMC article. Review.
References
-
- Auestad N, Korsak RA, Morrow JW, and Edmond J (1991). Fatty acid oxidation and ketogenesis by astrocytes in primary culture. Journal of neurochemistry 56, 1376–1386. - PubMed
-
- Blazquez C, Sanchez C, Velasco G, and Guzman M (1998). Role of carnitine palmitoyltransferase I in the control of ketogenesis in primary cultures of rat astrocytes. Journal of neurochemistry 71, 1597–1606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

