The Adenosine Analogue NITD008 has Potent Antiviral Activity against Human and Animal Caliciviruses
- PMID: 31151251
- PMCID: PMC6631109
- DOI: 10.3390/v11060496
The Adenosine Analogue NITD008 has Potent Antiviral Activity against Human and Animal Caliciviruses
Abstract
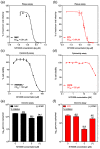
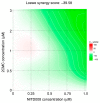
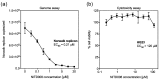
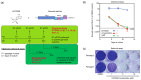
The widespread nature of calicivirus infections globally has a substantial impact on the health and well-being of humans and animals alike. Currently, the only vaccines approved against caliciviruses are for feline and rabbit-specific members of this group, and thus there is a growing effort towards the development of broad-spectrum antivirals for calicivirus infections. In this study, we evaluated the antiviral activity of the adenosine analogue NITD008 in vitro using three calicivirus model systems namely; feline calicivirus (FCV), murine norovirus (MNV), and the human norovirus replicon. We show that the nucleoside analogue (NA), NITD008, has limited toxicity and inhibits calicivirus replication in all three model systems with EC50 values of 0.94 μM, 0.91 µM, and 0.21 µM for MNV, FCV, and the Norwalk replicon, respectively. NITD008 has a similar level of potency to the most well-studied NA 2'-C-methylcytidine in vitro. Significantly, we also show that continual NITD008 treatment effectively cleared the Norwalk replicon from cells and treatment with 5 µM NITD008 was sufficient to completely prevent rebound. Given the potency displayed by NITD008 against several caliciviruses, we propose that this compound should be interrogated further to assess its effectiveness in vivo. In summary, we have added a potent NA to the current suite of antiviral compounds and provide a NA scaffold that could be further modified for therapeutic use against calicivirus infections.
Keywords: antivirals; caliciviruses; norovirus; nucleoside analogue; polymerase inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Potential Therapeutic Agents for Feline Calicivirus Infection.Viruses. 2018 Aug 16;10(8):433. doi: 10.3390/v10080433. Viruses. 2018. PMID: 30115859 Free PMC article.
-
Antiviral effects of grape seed extract against feline calicivirus, murine norovirus, and hepatitis A virus in model food systems and under gastric conditions.Food Microbiol. 2015 Dec;52:1-10. doi: 10.1016/j.fm.2015.05.011. Epub 2015 May 27. Food Microbiol. 2015. PMID: 26338111
-
Antiviral effect of theaflavins against caliciviruses.J Antibiot (Tokyo). 2017 Apr;70(4):443-447. doi: 10.1038/ja.2016.128. Epub 2016 Oct 19. J Antibiot (Tokyo). 2017. PMID: 27756911
-
Molecular virology of feline calicivirus.Vet Clin North Am Small Anim Pract. 2008 Jul;38(4):775-86, vii. doi: 10.1016/j.cvsm.2008.03.002. Vet Clin North Am Small Anim Pract. 2008. PMID: 18501277 Review.
-
[Calicivirus].Uirusu. 2011 Dec;61(2):193-203. doi: 10.2222/jsv.61.193. Uirusu. 2011. PMID: 22916566 Review. Japanese.
Cited by
-
Feline Calicivirus Virulent Systemic Disease: Clinical Epidemiology, Analysis of Viral Isolates and In Vitro Efficacy of Novel Antivirals in Australian Outbreaks.Viruses. 2021 Oct 9;13(10):2040. doi: 10.3390/v13102040. Viruses. 2021. PMID: 34696470 Free PMC article.
-
Current and Future Antiviral Strategies to Tackle Gastrointestinal Viral Infections.Microorganisms. 2021 Jul 27;9(8):1599. doi: 10.3390/microorganisms9081599. Microorganisms. 2021. PMID: 34442677 Free PMC article. Review.
-
Calicivirus Infection in Cats.Viruses. 2022 Apr 29;14(5):937. doi: 10.3390/v14050937. Viruses. 2022. PMID: 35632680 Free PMC article. Review.
-
Nitazoxanide protects cats from feline calicivirus infection and acts synergistically with mizoribine in vitro.Antiviral Res. 2020 Oct;182:104827. doi: 10.1016/j.antiviral.2020.104827. Epub 2020 Jun 21. Antiviral Res. 2020. PMID: 32579897 Free PMC article.
-
Broad spectrum antiviral nucleosides-Our best hope for the future.Annu Rep Med Chem. 2021;57:109-132. doi: 10.1016/bs.armc.2021.09.001. Epub 2021 Oct 29. Annu Rep Med Chem. 2021. PMID: 34728865 Free PMC article.
References
-
- Clarke I.N., Estes M.K., Green K.Y., Hansman G.S., Knowles N.J., Koopmans M.K., Matson D.O., Meyers G., Neill J.D., Radford A., et al. Caliciviridae. Elsevier; San Diego, CA, USA: 2012. pp. 977–986. 9th Report.
-
- Green K., Chanock R., Kapikian A. Human Caliciviruses. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2001. Fields virology; pp. 841–874.
-
- Pires S.M., Fischer-Walker C.L., Lanata C.F., Devleesschauwer B., Hall A.J., Kirk M.D., Duarte A.S., Black R.E., Angulo F.J. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS ONE. 2015;10:e0142927. doi: 10.1371/journal.pone.0142927. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

