The Cytoskeletal Protein Cyclase-Associated Protein 1 (CAP1) in Breast Cancer: Context-Dependent Roles in Both the Invasiveness and Proliferation of Cancer Cells and Underlying Cell Signals
- PMID: 31151140
- PMCID: PMC6600220
- DOI: 10.3390/ijms20112653
The Cytoskeletal Protein Cyclase-Associated Protein 1 (CAP1) in Breast Cancer: Context-Dependent Roles in Both the Invasiveness and Proliferation of Cancer Cells and Underlying Cell Signals
Abstract
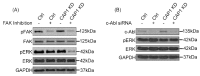
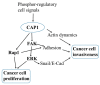
As a conserved actin-regulating protein, CAP (adenylyl Cyclase-Associated Protein) functions to facilitate the rearrangement of the actin cytoskeleton. The ubiquitously expressed isoform CAP1 drives mammalian cell migration, and accordingly, most studies on the involvement of CAP1 in human cancers have largely been based on the rationale that up-regulated CAP1 will stimulate cancer cell migration and invasiveness. While findings from some studies reported so far support this case, lines of evidence largely from our recent studies point to a more complex and profound role for CAP1 in the invasiveness of cancer cells, where the potential activation of cell adhesion signaling is believed to play a key role. Moreover, CAP1 was also found to control proliferation in breast cancer cells, through the regulation of ERK (External signal-Regulated Kinase). Alterations in the activities of FAK (Focal Adhesion Kinase) and ERK from CAP1 depletion that are consistent to the opposite adhesion and proliferation phenotypes were detected in the metastatic and non-metastatic breast cancer cells. In this review, we begin with the overview of the literature on CAP, by highlighting the molecular functions of mammalian CAP1 in regulating the actin cytoskeleton and cell adhesion. We will next discuss the role of the FAK/ERK axis, and possibly Rap1, in mediating CAP1 signals to control breast cancer cell adhesion, invasiveness, and proliferation, largely based on our latest findings. Finally, we will discuss the relevance of these novel mechanistic insights to ultimately realizing the translational potential of CAP1 in targeted therapeutics for breast cancer.
Keywords: CAP1; ERK; FAK; breast cancer; cell adhesion; cell invasiveness; cell proliferation; the actin cytoskeleton.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsor had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

Similar articles
-
Phosphorylation Regulates CAP1 (Cyclase-Associated Protein 1) Functions in the Motility and Invasion of Pancreatic Cancer Cells.Sci Rep. 2019 Mar 20;9(1):4925. doi: 10.1038/s41598-019-41346-3. Sci Rep. 2019. PMID: 30894654 Free PMC article.
-
Mammalian adenylyl cyclase-associated protein 1 (CAP1) regulates cofilin function, the actin cytoskeleton, and cell adhesion.J Biol Chem. 2013 Jul 19;288(29):20966-20977. doi: 10.1074/jbc.M113.484535. Epub 2013 Jun 4. J Biol Chem. 2013. PMID: 23737525 Free PMC article.
-
CAP1 (Cyclase-Associated Protein 1) Exerts Distinct Functions in the Proliferation and Metastatic Potential of Breast Cancer Cells Mediated by ERK.Sci Rep. 2016 May 13;6:25933. doi: 10.1038/srep25933. Sci Rep. 2016. PMID: 27173014 Free PMC article.
-
Adenylyl Cyclase-Associated Protein 1: Structure, Regulation, and Participation in Cellular Processes.Biochemistry (Mosc). 2018 Jan;83(1):45-53. doi: 10.1134/S0006297918010066. Biochemistry (Mosc). 2018. PMID: 29534668 Review.
-
The role of cyclase-associated protein in regulating actin filament dynamics - more than a monomer-sequestration factor.J Cell Sci. 2013 Aug 1;126(Pt 15):3249-58. doi: 10.1242/jcs.128231. J Cell Sci. 2013. PMID: 23908377 Free PMC article. Review.
Cited by
-
miRNA-451 regulates rhesus choroid-retinal endothelial cell function and proteome profile.Int J Ophthalmol. 2022 Jun 18;15(6):894-904. doi: 10.18240/ijo.2022.06.06. eCollection 2022. Int J Ophthalmol. 2022. PMID: 35814901 Free PMC article.
-
BAIAP2L1 accelerates breast cancer progression and chemoresistance by activating AKT signaling through binding with ribosomal protein L3.Cancer Sci. 2023 Mar;114(3):764-780. doi: 10.1111/cas.15632. Epub 2022 Dec 1. Cancer Sci. 2023. PMID: 36308067 Free PMC article.
-
Adipo-oncology: adipocyte-derived factors govern engraftment, survival, and progression of metastatic cancers.Cell Commun Signal. 2024 Jan 18;22(1):52. doi: 10.1186/s12964-024-01474-4. Cell Commun Signal. 2024. PMID: 38238841 Free PMC article. Review.
-
The Significant Role of the Microfilament System in Tumors.Front Oncol. 2021 Mar 17;11:620390. doi: 10.3389/fonc.2021.620390. eCollection 2021. Front Oncol. 2021. PMID: 33816252 Free PMC article. Review.
-
Oligomerization Affects the Ability of Human Cyclase-Associated Proteins 1 and 2 to Promote Actin Severing by Cofilins.Int J Mol Sci. 2019 Nov 12;20(22):5647. doi: 10.3390/ijms20225647. Int J Mol Sci. 2019. PMID: 31718088 Free PMC article.
References
-
- Field J., Vojtek A., Ballester R., Bolger G., Colicelli J., Ferguson K., Gerst J., Kataoka T., Michaeli T., Powers S., et al. Cloning and characterization of cap, the s. Cerevisiae gene encoding the 70 kd adenylyl cyclase-associated protein. Cell. 1990;61:319–327. doi: 10.1016/0092-8674(90)90812-S. - DOI - PubMed
-
- Nishida Y., Shima F., Sen H., Tanaka Y., Yanagihara C., Yamawaki-Kataoka Y., Kariya K., Kataoka T. Coiled-coil interaction of n-terminal 36 residues of cyclase-associated protein with adenylyl cyclase is sufficient for its function in saccharomyces cerevisiae ras pathway. J. Biol. Chem. 1998;273:28019–28024. doi: 10.1074/jbc.273.43.28019. - DOI - PubMed
-
- Shima F., Okada T., Kido M., Sen H., Tanaka Y., Tamada M., Hu C.D., Yamawaki-Kataoka Y., Kariya K., Kataoka T. Association of yeast adenylyl cyclase with cyclase-associated protein cap forms a second ras-binding site which mediates its ras-dependent activation. Mol. Cell. Biol. 2000;20:26–33. doi: 10.1128/MCB.20.1.26-33.2000. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

