The cAMP Pathway Amplifies Early MyD88-Dependent and Type I Interferon-Independent LPS-Induced Interleukin-10 Expression in Mouse Macrophages
- PMID: 31148944
- PMCID: PMC6501241
- DOI: 10.1155/2019/3451461
The cAMP Pathway Amplifies Early MyD88-Dependent and Type I Interferon-Independent LPS-Induced Interleukin-10 Expression in Mouse Macrophages
Abstract
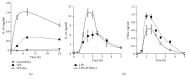
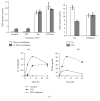
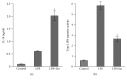
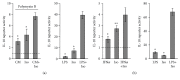
Interleukin-10 (IL-10) is a key anti-inflammatory cytokine, secreted by macrophages and other immune cells to attenuate inflammation. Autocrine type I interferons (IFNs) largely mediate the delayed expression of IL-10 by LPS-stimulated macrophages. We have previously shown that IL-10 is synergistically expressed in macrophages following a costimulus of a TLR agonist and cAMP. We now show that the cAMP pathway directly upregulates IL-10 transcription and plays an important permissive and synergistic role in early, but not late, LPS-stimulated IL-10 mRNA and protein expression in mouse macrophages and in a mouse septic shock model. Our results suggest that the loss of synergism is not due to desensitization of the cAMP inducing signal, and it is not mediated by a positive crosstalk between the cAMP and type I IFN pathways. First, cAMP elevation in LPS-treated cells decreased the secretion of type I IFN. Second, autocrine/paracrine type I IFNs induce IL-10 promoter reporter activity only additively, but not synergistically, with the cAMP pathway. IL-10 promoter reporter activity was synergistically induced by cAMP elevation in macrophages stimulated by an agonist of either TLR4, TLR2/6, or TLR7, receptors which signal via MyD88, but not by an agonist of TLR3 which signals independently of MyD88. Moreover, MyD88 knockout largely reduced the synergistic IL-10 expression, indicating that MyD88 is required for the synergism displayed by LPS with cAMP. This report delineates the temporal regulation of early cAMP-accelerated vs. late type I IFN-dependent IL-10 transcription in LPS-stimulated murine macrophages that can limit inflammation at its onset.
Figures

Similar articles
-
Exclusive Temporal Stimulation of IL-10 Expression in LPS-Stimulated Mouse Macrophages by cAMP Inducers and Type I Interferons.Front Immunol. 2019 Aug 6;10:1788. doi: 10.3389/fimmu.2019.01788. eCollection 2019. Front Immunol. 2019. PMID: 31447835 Free PMC article.
-
Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells.J Pineal Res. 2012 Nov;53(4):325-34. doi: 10.1111/j.1600-079X.2012.01002.x. Epub 2012 Apr 27. J Pineal Res. 2012. PMID: 22537289
-
Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages.J Immunol. 2002 Nov 15;169(10):5874-80. doi: 10.4049/jimmunol.169.10.5874. J Immunol. 2002. PMID: 12421970
-
Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways.J Exp Med. 2007 Jan 22;204(1):141-52. doi: 10.1084/jem.20061440. Epub 2007 Jan 16. J Exp Med. 2007. PMID: 17227910 Free PMC article.
-
The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol.Eur J Immunol. 2001 Aug;31(8):2448-57. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. Eur J Immunol. 2001. PMID: 11500829
Cited by
-
Aminoclay Nanoparticles Induce Anti-Inflammatory Dendritic Cells to Attenuate LPS-Elicited Pro-Inflammatory Immune Responses.Molecules. 2022 Dec 9;27(24):8743. doi: 10.3390/molecules27248743. Molecules. 2022. PMID: 36557876 Free PMC article.
-
NLRP3 Inflammasome Activation by MicroRNA-495 Promoter Methylation May Contribute to the Progression of Acute Lung Injury.Mol Ther Nucleic Acids. 2019 Dec 6;18:801-814. doi: 10.1016/j.omtn.2019.08.028. Epub 2019 Oct 3. Mol Ther Nucleic Acids. 2019. PMID: 31734560 Free PMC article.
-
Type I Interferon, Induced by Adenovirus or Adenoviral Vector Infection, Regulates the Cytokine Response to Lipopolysaccharide in a Macrophage Type-Specific Manner.J Innate Immun. 2024;16(1):226-247. doi: 10.1159/000538282. Epub 2024 Mar 25. J Innate Immun. 2024. PMID: 38527452 Free PMC article.
-
Changes in the expression of interleukin-10 in myocardial infarction and its relationship with macrophage activation and cell apoptosis.Ann Transl Med. 2020 May;8(10):643. doi: 10.21037/atm-20-3349. Ann Transl Med. 2020. PMID: 32566580 Free PMC article.
-
Exclusive Temporal Stimulation of IL-10 Expression in LPS-Stimulated Mouse Macrophages by cAMP Inducers and Type I Interferons.Front Immunol. 2019 Aug 6;10:1788. doi: 10.3389/fimmu.2019.01788. eCollection 2019. Front Immunol. 2019. PMID: 31447835 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

