Phosphatidylinositol 4,5-bisphosphate drives Ca2+-independent membrane penetration by the tandem C2 domain proteins synaptotagmin-1 and Doc2β
- PMID: 31147445
- PMCID: PMC6635456
- DOI: 10.1074/jbc.RA119.007929
Phosphatidylinositol 4,5-bisphosphate drives Ca2+-independent membrane penetration by the tandem C2 domain proteins synaptotagmin-1 and Doc2β
Abstract
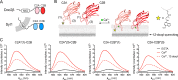
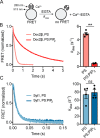
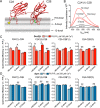
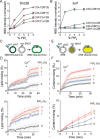
Exocytosis mediates the release of neurotransmitters and hormones from neurons and neuroendocrine cells. Tandem C2 domain proteins in the synaptotagmin (syt) and double C2 domain (Doc2) families regulate exocytotic membrane fusion via direct interactions with Ca2+ and phospholipid bilayers. Syt1 is a fast-acting, low-affinity Ca2+ sensor that penetrates membranes upon binding Ca2+ to trigger synchronous vesicle fusion. The closely related Doc2β is a slow-acting, high-affinity Ca2+ sensor that triggers spontaneous and asynchronous vesicle fusion, but whether it also penetrates membranes is unknown. Both syt1 and Doc2β bind the dynamically regulated plasma membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2), but it is unclear whether PIP2 serves only as a membrane contact or enables specialized membrane-binding modes by these Ca2+ sensors. Furthermore, it has been shown that PIP2 uncaging can trigger rapid, syt1-dependent exocytosis in the absence of Ca2+ influx, suggesting that current models for the action of these Ca2+ sensors are incomplete. Here, using a series of steady-state and time-resolved fluorescence measurements, we show that Doc2β, like syt1, penetrates membranes in a Ca2+-dependent manner. Unexpectedly, we observed that PIP2 can drive membrane penetration by both syt1 and Doc2β in the absence of Ca2+, providing a plausible mechanism for Ca2+-independent, PIP2-dependent exocytosis. Quantitative measurements of penetration depth revealed that, in the presence of Ca2+, PIP2 drives Doc2β, but not syt1, substantially deeper into the membrane, defining a biophysical regulatory mechanism specific to this high-affinity Ca2+ sensor. Our results provide evidence of a novel role for PIP2 in regulating, and under some circumstances triggering, exocytosis.
Keywords: calcium sensor; calcium-binding protein; exocytosis; membrane biophysics; membrane protein; phosphatidylinositol (4,5)-bisphosphate (PIP2); poly-anionic phospholipid; synapse; synaptotagmin; tandem C2 domain protein.
© 2019 Bradberry et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Allosteric stabilization of calcium and phosphoinositide dual binding engages several synaptotagmins in fast exocytosis.Elife. 2022 Aug 5;11:e74810. doi: 10.7554/eLife.74810. Elife. 2022. PMID: 35929728 Free PMC article.
-
Structural elements that underlie Doc2β function during asynchronous synaptic transmission.Proc Natl Acad Sci U S A. 2015 Aug 4;112(31):E4316-25. doi: 10.1073/pnas.1502288112. Epub 2015 Jul 20. Proc Natl Acad Sci U S A. 2015. PMID: 26195798 Free PMC article.
-
Mutations that disrupt Ca²⁺-binding activity endow Doc2β with novel functional properties during synaptic transmission.Mol Biol Cell. 2014 Feb;25(4):481-94. doi: 10.1091/mbc.E13-10-0571. Epub 2013 Dec 19. Mol Biol Cell. 2014. PMID: 24356452 Free PMC article.
-
Models of synaptotagmin-1 to trigger Ca2+ -dependent vesicle fusion.FEBS Lett. 2018 Nov;592(21):3480-3492. doi: 10.1002/1873-3468.13193. Epub 2018 Jul 30. FEBS Lett. 2018. PMID: 30004579 Review.
-
Exocytosis and synaptic vesicle function.Compr Physiol. 2014 Jan;4(1):149-75. doi: 10.1002/cphy.c130021. Compr Physiol. 2014. PMID: 24692137 Review.
Cited by
-
Synaptotagmin 1 oligomerization via the juxtamembrane linker regulates spontaneous and evoked neurotransmitter release.Proc Natl Acad Sci U S A. 2021 Nov 30;118(48):e2113859118. doi: 10.1073/pnas.2113859118. Proc Natl Acad Sci U S A. 2021. PMID: 34810248 Free PMC article.
-
Exocytosis Proteins: Typical and Atypical Mechanisms of Action in Skeletal Muscle.Front Endocrinol (Lausanne). 2022 Jun 14;13:915509. doi: 10.3389/fendo.2022.915509. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35774142 Free PMC article. Review.
-
SNARE complex alters the interactions of the Ca2+ sensor synaptotagmin 1 with lipid bilayers.Biophys J. 2021 Feb 16;120(4):642-661. doi: 10.1016/j.bpj.2020.12.025. Epub 2021 Jan 14. Biophys J. 2021. PMID: 33453271 Free PMC article.
-
Cholesterol modulates the structural dynamics of the paddle motif loop of KvAP voltage sensor.Curr Res Struct Biol. 2024 Mar 6;7:100137. doi: 10.1016/j.crstbi.2024.100137. eCollection 2024. Curr Res Struct Biol. 2024. PMID: 38500801 Free PMC article.
-
Phosphatidylinositol(4,5)bisphosphate: diverse functions at the plasma membrane.Essays Biochem. 2020 Sep 23;64(3):513-531. doi: 10.1042/EBC20200041. Essays Biochem. 2020. PMID: 32844214 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

