The interaction of hepatitis B virus with the ubiquitin proteasome system in viral replication and associated pathogenesis
- PMID: 31146743
- PMCID: PMC6543661
- DOI: 10.1186/s12985-019-1183-z
The interaction of hepatitis B virus with the ubiquitin proteasome system in viral replication and associated pathogenesis
Abstract
Background: The ubiquitin proteasome system (UPS) regulates the expression levels of cellular proteins by ubiquitination of protein substrates followed by their degradation via the proteasome. As a highly conserved cellular degradation mechanism, the UPS affects a variety of biological processes and participates in viral propagation.
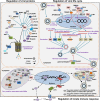
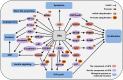
Main body: During hepatitis B virus (HBV) infection, the UPS is shown to act as a double-edged sword in viral pathogenesis. On the one hand, the UPS acts as a host defense mechanism to selectively recognize HBV proteins as well as special cellular proteins that favor the viral life cycle and induces their ubiquitin-dependent proteasomal degradation to limit HBV infection. On the other hand, the HBV has evolved to subvert the UPS function for its own advantage. Moreover, in the infected hepatocytes, certain cellular proteins that are dependent on the UPS are involved in abnormal biological processes which are mediated by HBV.
Conclusion: The molecular interaction of HBV with the UPS to modulate viral propagation and pathogenesis is summarized in the review. Considering the important role of the UPS in HBV infection, a better understanding of the HBV-UPS interaction could provide novel insight into the mechanisms that are involved in viral replication and pathogenesis and help to develop potential treatment strategies targeting the UPS.
Keywords: Hepatitis B virus; Pathogenesis; Proteasome; Replication; Ubiquitin.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Hepatitis B virus HBx protein interactions with the ubiquitin proteasome system.Viruses. 2014 Nov 24;6(11):4683-702. doi: 10.3390/v6114683. Viruses. 2014. PMID: 25421893 Free PMC article. Review.
-
Interplay between the virus and the ubiquitin-proteasome system: molecular mechanism of viral pathogenesis.Curr Opin Virol. 2016 Apr;17:1-10. doi: 10.1016/j.coviro.2015.09.005. Epub 2015 Sep 29. Curr Opin Virol. 2016. PMID: 26426962 Free PMC article. Review.
-
Hepatitis C virus core protein inhibits E6AP expression via DNA methylation to escape from ubiquitin-dependent proteasomal degradation.Cancer Lett. 2016 Sep 28;380(1):59-68. doi: 10.1016/j.canlet.2016.06.008. Epub 2016 Jun 15. Cancer Lett. 2016. PMID: 27317649
-
Human stem cell-derived hepatocytes as a model for hepatitis B virus infection, spreading and virus-host interactions.J Hepatol. 2017 Mar;66(3):494-503. doi: 10.1016/j.jhep.2016.10.009. Epub 2016 Oct 14. J Hepatol. 2017. PMID: 27746336 Free PMC article.
-
Inhibition of hepatitis B virus replication by cIAP2 involves accelerating the ubiquitin-proteasome-mediated destruction of polymerase.J Virol. 2011 Nov;85(21):11457-67. doi: 10.1128/JVI.00879-11. Epub 2011 Aug 24. J Virol. 2011. PMID: 21865390 Free PMC article.
Cited by
-
Hepatitis B virus core protein stabilizes RANGAP1 to upregulate KDM2A and facilitate hepatocarcinogenesis.Cell Oncol (Dordr). 2024 Apr;47(2):639-655. doi: 10.1007/s13402-023-00889-4. Epub 2023 Oct 17. Cell Oncol (Dordr). 2024. PMID: 37845585
-
Insight into the mechanisms regulating liver cancer stem cells by hepatitis B virus X protein.Infect Agent Cancer. 2024 Nov 11;19(1):56. doi: 10.1186/s13027-024-00618-y. Infect Agent Cancer. 2024. PMID: 39529119 Free PMC article. Review.
-
Role of Virally-Encoded Deubiquitinating Enzymes in Regulation of the Virus Life Cycle.Int J Mol Sci. 2021 Apr 23;22(9):4438. doi: 10.3390/ijms22094438. Int J Mol Sci. 2021. PMID: 33922750 Free PMC article. Review.
-
Upregulated Proteasome Subunits in COVID-19 Patients: A Link with Hypoxemia, Lymphopenia and Inflammation.Biomolecules. 2022 Mar 13;12(3):442. doi: 10.3390/biom12030442. Biomolecules. 2022. PMID: 35327634 Free PMC article.
-
Calcium signaling in hepatitis B virus infection and its potential as a therapeutic target.Cell Commun Signal. 2021 Aug 6;19(1):82. doi: 10.1186/s12964-021-00762-7. Cell Commun Signal. 2021. PMID: 34362380 Free PMC article. Review.
References
-
- Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

