Myo1f, an Unconventional Long-Tailed Myosin, Is a New Partner for the Adaptor 3BP2 Involved in Mast Cell Migration
- PMID: 31143189
- PMCID: PMC6521229
- DOI: 10.3389/fimmu.2019.01058
Myo1f, an Unconventional Long-Tailed Myosin, Is a New Partner for the Adaptor 3BP2 Involved in Mast Cell Migration
Abstract
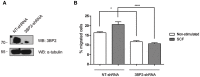
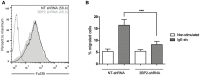
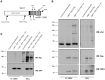
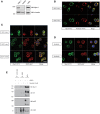
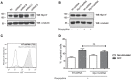
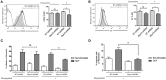
Mast cell chemotaxis is essential for cell recruitment to target tissues, where these cells play an important role in adaptive and innate immunity. Stem cell factor (SCF) is a major chemoattractant for mast cells. SCF binds to the KIT receptor, thereby triggering tyrosine phosphorylation in the cytoplasmic domain and resulting in docking sites for SH2 domain-containing molecules, such as Lyn and Fyn, and the subsequent activation of the small GTPases Rac that are responsible for cytoskeletal reorganization and mast cell migration. In previous works we have reported the role of 3BP2, an adaptor molecule, in mast cells. 3BP2 silencing reduces FcεRI-dependent degranulation, by targeting Lyn and Syk phosphorylation, as well as SCF-dependent cell survival. This study examines its role in SCF-dependent migration and reveals that 3BP2 silencing in human mast cell line (LAD2) impairs cell migration due to SCF and IgE. In that context we found that 3BP2 silencing decreases Rac-2 and Cdc42 GTPase activity. Furthermore, we identified Myo1f, an unconventional type-I myosin, as a new partner for 3BP2. This protein, whose functions have been described as critical for neutrophil migration, remained elusive in mast cells. Myo1f is expressed in mast cells and colocalizes with cortical actin ring. Interestingly, Myo1f-3BP2 interaction is modulated by KIT signaling. Moreover, SCF dependent adhesion and migration through fibronectin is decreased after Myo1f silencing. Furthermore, Myo1f silencing leads to downregulation of β1 and β7 integrins on the mast cell membrane. Overall, Myo1f is a new 3BP2 ligand that connects the adaptor to actin cytoskeleton and both molecules are involved in SCF dependent mast cell migration.
Keywords: KIT signaling; adaptor molecules; cell migration and adhesion; cytoske leton; mast cells; unconventional myosins.
Figures
Similar articles
-
MYO1F Regulates IgE and MRGPRX2-Dependent Mast Cell Exocytosis.J Immunol. 2021 May 15;206(10):2277-2289. doi: 10.4049/jimmunol.2001211. Epub 2021 May 3. J Immunol. 2021. PMID: 33941653 Free PMC article.
-
The adaptor 3BP2 is required for KIT receptor expression and human mast cell survival.J Immunol. 2015 May 1;194(9):4309-18. doi: 10.4049/jimmunol.1402887. Epub 2015 Mar 25. J Immunol. 2015. PMID: 25810396 Free PMC article.
-
Involvement of Fyn kinase in Kit and integrin-mediated Rac activation, cytoskeletal reorganization, and chemotaxis of mast cells.Blood. 2007 May 1;109(9):3679-86. doi: 10.1182/blood-2006-11-057315. Epub 2007 Jan 9. Blood. 2007. PMID: 17213284 Free PMC article.
-
Adaptor protein 3BP2 and cherubism.Curr Med Chem. 2008;15(6):549-54. doi: 10.2174/092986708783769795. Curr Med Chem. 2008. PMID: 18336269 Review.
-
The c-kit receptor, stem cell factor, and mast cells. What each is teaching us about the others.Am J Pathol. 1993 Apr;142(4):965-74. Am J Pathol. 1993. PMID: 7682764 Free PMC article. Review.
Cited by
-
Long-Tailed Unconventional Class I Myosins in Health and Disease.Int J Mol Sci. 2020 Apr 7;21(7):2555. doi: 10.3390/ijms21072555. Int J Mol Sci. 2020. PMID: 32272642 Free PMC article. Review.
-
Myo1f has an essential role in γδT intraepithelial lymphocyte adhesion and migration.Front Immunol. 2023 May 3;14:1041079. doi: 10.3389/fimmu.2023.1041079. eCollection 2023. Front Immunol. 2023. PMID: 37207213 Free PMC article.
-
E3-ubiquitin ligases and recent progress in osteoimmunology.Front Immunol. 2023 Feb 23;14:1120710. doi: 10.3389/fimmu.2023.1120710. eCollection 2023. Front Immunol. 2023. PMID: 36911671 Free PMC article. Review.
-
MYO1F regulates antifungal immunity by regulating acetylation of microtubules.Proc Natl Acad Sci U S A. 2021 Jul 27;118(30):e2100230118. doi: 10.1073/pnas.2100230118. Proc Natl Acad Sci U S A. 2021. PMID: 34301894 Free PMC article.
-
MYO1F Regulates IgE and MRGPRX2-Dependent Mast Cell Exocytosis.J Immunol. 2021 May 15;206(10):2277-2289. doi: 10.4049/jimmunol.2001211. Epub 2021 May 3. J Immunol. 2021. PMID: 33941653 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

