Characteristics of mucosa-associated gut microbiota during treatment in Crohn's disease
- PMID: 31143071
- PMCID: PMC6526154
- DOI: 10.3748/wjg.v25.i18.2204
Characteristics of mucosa-associated gut microbiota during treatment in Crohn's disease
Abstract
Background: The dysbiosis of the gut microbiome is evident in Crohn's disease (CD) compared with healthy controls (HC), although the alterations from active CD to remission after treatment are unclear.
Aim: To characterize the mucosa-associated gut microbiota in patients with CD before and after the induction therapy.
Methods: The basic information was collected from the subjects and the CD activity index (CDAI) was calculated in patients. A 16S rRNA sequencing approach was applied to determine the structures of microbial communities in mucosal samples including the terminal ileal, ascending colon, descending colon and rectum. The composition and function of mucosa-associated gut microbiota were compared between samples from the same cohort of patients before and after treatment. Differential taxa were identified to calculate the microbial dysbiosis index (MDI) and the correlation between MDI and CDAI was analyzed using Pearson correlation test. Predictive functional profiling of microbial communities was obtained with PICRUSt.
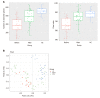
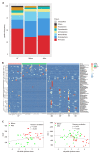
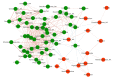
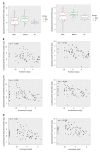
Results: There were no significant differences in microbial richness among the four anatomical sites in individuals. Compared to active disease, the alpha diversity of CD in remission was increased towards the level of HC compared to the active stage. The principal coordinate analysis revealed that samples of active CD were clearly separated from those in remission, which clustered close to HC. Sixty-five genera were identified as differentially abundant between active and quiescent CD, with a loss of Fusobacterium and a gain of potential beneficial bacteria including Lactobacillus, Akkermansia, Roseburia, Ruminococcus and Lachnospira after the induction of remission. The combination of these taxa into a MDI showed a positive correlation with clinical disease severity and a negative correlation with species richness. The increased capacity for the inferred pathways including Lipopolysaccharide biosynthesis and Lipopolysaccharide biosynthesis proteins in patients before treatment negatively correlated with the abundance of Roseburia, Ruminococcus and Lachnospira.
Conclusion: The dysbiosis of mucosa-associated microbiota was associated with the disease phenotype and may become a potential diagnostic tool for the recurrence of disease.
Keywords: 16S rRNA sequence; Active; Crohn’s disease; Mucosa-associated gut microbiota; Remission.
Conflict of interest statement
Conflict-of-interest statement: To the best of our knowledge, no conflict of interest exists.
Figures
Similar articles
-
Characteristics of Faecal Microbiota in Paediatric Crohn's Disease and Their Dynamic Changes During Infliximab Therapy.J Crohns Colitis. 2018 Feb 28;12(3):337-346. doi: 10.1093/ecco-jcc/jjx153. J Crohns Colitis. 2018. PMID: 29194468
-
Structural robustness of the gut mucosal microbiota is associated with Crohn's disease remission after surgery.Gut. 2016 Jun;65(6):954-62. doi: 10.1136/gutjnl-2015-309184. Epub 2015 Dec 1. Gut. 2016. PMID: 26628508 Free PMC article. Clinical Trial.
-
Inflammation-related differences in mucosa-associated microbiota and intestinal barrier function in colonic Crohn's disease.Am J Physiol Gastrointest Liver Physiol. 2018 Sep 1;315(3):G420-G431. doi: 10.1152/ajpgi.00411.2017. Epub 2018 May 31. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29848021
-
The Microbiome in Crohn's Disease: Role in Pathogenesis and Role of Microbiome Replacement Therapies.Gastroenterol Clin North Am. 2017 Sep;46(3):481-492. doi: 10.1016/j.gtc.2017.05.004. Epub 2017 Jul 19. Gastroenterol Clin North Am. 2017. PMID: 28838410 Review.
-
Microbiota and mucosal defense in IBD: an update.Expert Rev Gastroenterol Hepatol. 2019 Oct;13(10):963-976. doi: 10.1080/17474124.2019.1671822. Epub 2019 Oct 11. Expert Rev Gastroenterol Hepatol. 2019. PMID: 31603356 Review.
Cited by
-
Vital members in the gut microbiotas altered by two probiotic Bifidobacterium strains against liver damage in rats.BMC Microbiol. 2020 Jun 5;20(1):144. doi: 10.1186/s12866-020-01827-2. BMC Microbiol. 2020. PMID: 32503418 Free PMC article.
-
Vital Members in the More Dysbiotic Oropharyngeal Microbiotas in H7N9-Infected Patients.Front Med (Lausanne). 2020 Aug 11;7:396. doi: 10.3389/fmed.2020.00396. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32850904 Free PMC article.
-
Cultivating complexity: Advancements in establishing in vitro models for the mucus-adhering gut microbiota.Microb Biotechnol. 2024 Oct;17(10):e70036. doi: 10.1111/1751-7915.70036. Microb Biotechnol. 2024. PMID: 39435730 Free PMC article. Review.
-
Gut Microbiota Parameters Potentially Useful in Clinical Perspective.Microorganisms. 2021 Nov 22;9(11):2402. doi: 10.3390/microorganisms9112402. Microorganisms. 2021. PMID: 34835527 Free PMC article. Review.
-
Diversity of the microbiota communities found in the various regions of the intestinal tract in healthy individuals and inflammatory bowel diseases.Front Immunol. 2023 Nov 2;14:1242242. doi: 10.3389/fimmu.2023.1242242. eCollection 2023. Front Immunol. 2023. PMID: 38022505 Free PMC article. Review.
References
-
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2018;390:2769–2778. - PubMed
-
- Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670–1689. - PubMed
-
- Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, Amil Dias J, Barabino A, Braegger CP, Bronsky J, Buderus S, Martín-de-Carpi J, De Ridder L, Fagerberg UL, Hugot JP, Kierkus J, Kolacek S, Koletzko S, Lionetti P, Miele E, Navas López VM, Paerregaard A, Russell RK, Serban DE, Shaoul R, Van Rheenen P, Veereman G, Weiss B, Wilson D, Dignass A, Eliakim A, Winter H, Turner D European Crohn's and Colitis Organisation; European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014;8:1179–1207. - PubMed
-
- Fishman SJ, Feins NR, D'Amoto RJ, Folkman J. Thalidomide for Crohn's disease. Gastroenterology. 2000;119:596. - PubMed
-
- de Souza HS, Fiocchi C. Immunopathogenesis of IBD: Current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

