Neural Stem Cells of the Subventricular Zone Contribute to Neuroprotection of the Corpus Callosum after Cuprizone-Induced Demyelination
- PMID: 31138656
- PMCID: PMC6616285
- DOI: 10.1523/JNEUROSCI.0227-18.2019
Neural Stem Cells of the Subventricular Zone Contribute to Neuroprotection of the Corpus Callosum after Cuprizone-Induced Demyelination
Abstract
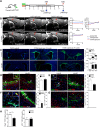
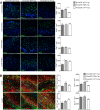
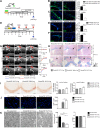
Myelin loss occurring in demyelinating diseases, including multiple sclerosis, is the leading cause of long-lasting neurological disability in adults. While endogenous remyelination, driven by resident oligodendrocyte precursor cells (OPCs), might partially compensate myelin loss in the early phases of demyelinating disorders, this spontaneous reparative potential fails at later stages. To investigate the cellular mechanisms sustaining endogenous remyelination in demyelinating disorders, we focused our attention on endogenous neural precursor cells (eNPCs) located within the subventricular zone (SVZ) since this latter area is considered one of the primary sources of new OPCs in the adult forebrain. First, we fate mapped SVZ-eNPCs in cuprizone-induced demyelination and found that SVZ endogenous neural stem/precursor cells are recruited during the remyelination phase to the corpus callosum (CC) and are capable of forming new oligodendrocytes. When we ablated SVZ-derived eNPCs during cuprizone-induced demyelination in female mice, the animals displayed reduced numbers of oligodendrocytes within the lesioned CC. Although this reduction in oligodendrocytes did not impact the ensuing remyelination, eNPC-ablated mice experienced increased axonal loss. Our results indicate that, in toxic models of demyelination, SVZ-derived eNPCs contribute to support axonal survival.SIGNIFICANCE STATEMENT One of the significant challenges in MS research is to understand the detrimental mechanisms leading to the failure of CNS tissue regeneration during disease progression. One possible explanation is the inability of recruited oligodendrocyte precursor cells (OPCs) to complete remyelination and to sustain axonal survival. The contribution of endogenous neural precursor cells (eNPCs) located in the subventricular zone (SVZ) to generate new OPCs in the lesion site has been debated. Using transgenic mice to fate map and to selectively kill SVZ-derived eNPCs in the cuprizone demyelination model, we observed migration of SVZ-eNPCs after injury and their contribution to oligodendrogenesis and axonal survival. We found that eNPCs are dispensable for remyelination but protect partially from increased axonal loss.
Keywords: ablation; cuprizone; multiple sclerosis; neural stem cells; sub ventricular zone; transgenic mice.
Copyright © 2019 the authors.
Figures


Similar articles
-
Multimodal imaging of subventricular zone neural stem/progenitor cells in the cuprizone mouse model reveals increased neurogenic potential for the olfactory bulb pathway, but no contribution to remyelination of the corpus callosum.Neuroimage. 2014 Feb 1;86:99-110. doi: 10.1016/j.neuroimage.2013.07.080. Epub 2013 Aug 7. Neuroimage. 2014. PMID: 23933305
-
Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination.J Neurosci. 2014 Oct 15;34(42):14128-46. doi: 10.1523/JNEUROSCI.3491-13.2014. J Neurosci. 2014. PMID: 25319708 Free PMC article.
-
A Morphological and Behavioral Study of Demyelination and Remyelination in the Cuprizone Model: Insights into APLNR and NG2+ Cell Dynamics.Int J Mol Sci. 2024 Dec 3;25(23):13011. doi: 10.3390/ijms252313011. Int J Mol Sci. 2024. PMID: 39684720 Free PMC article.
-
Oligodendrogenesis from neural stem cells: perspectives for remyelinating strategies.Int J Dev Neurosci. 2013 Nov;31(7):692-700. doi: 10.1016/j.ijdevneu.2013.01.004. Epub 2013 Jan 20. Int J Dev Neurosci. 2013. PMID: 23340483 Review.
-
Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor.Brain Res Rev. 2011 Jun 24;67(1-2):147-56. doi: 10.1016/j.brainresrev.2011.01.001. Epub 2011 Jan 12. Brain Res Rev. 2011. PMID: 21236296 Free PMC article. Review.
Cited by
-
Wnt/β-Catenin Signaling Promotes Differentiation of Ischemia-Activated Adult Neural Stem/Progenitor Cells to Neuronal Precursors.Front Neurosci. 2021 Feb 25;15:628983. doi: 10.3389/fnins.2021.628983. eCollection 2021. Front Neurosci. 2021. PMID: 33716653 Free PMC article.
-
Endogenous Neural Stem Cell Mediated Oligodendrogenesis in the Adult Mammalian Brain.Cells. 2022 Jul 2;11(13):2101. doi: 10.3390/cells11132101. Cells. 2022. PMID: 35805185 Free PMC article. Review.
-
A cell type-specific expression map of NCoR1 and SMRT transcriptional co-repressors in the mouse brain.J Comp Neurol. 2020 Sep 1;528(13):2218-2238. doi: 10.1002/cne.24886. Epub 2020 Feb 24. J Comp Neurol. 2020. PMID: 32072640 Free PMC article.
-
Regulation of microglia function by neural stem cells.Front Cell Neurosci. 2023 Mar 1;17:1130205. doi: 10.3389/fncel.2023.1130205. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36937181 Free PMC article. Review.
-
Calcium Channels in Adult Brain Neural Stem Cells and in Glioblastoma Stem Cells.Front Cell Neurosci. 2020 Nov 13;14:600018. doi: 10.3389/fncel.2020.600018. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33281564 Free PMC article. Review.
References
-
- Bacigaluppi M, Russo GL, Peruzzotti-Jametti L, Rossi S, Sandrone S, Butti E, De Ceglia R, Bergamaschi A, Motta C, Gallizioli M, Studer V, Colombo E, Farina C, Comi G, Politi LS, Muzio L, Villani C, Invernizzi RW, Hermann DM, Centonze D, et al. (2016) Neural stem cell transplantation induces stroke recovery by upregulating glutamate transporter GLT-1 in astrocytes. J Neurosci 36:10529–10544. 10.1523/JNEUROSCI.1643-16.2016 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
