Ablation of Hepatocyte Smad1, Smad5, and Smad8 Causes Severe Tissue Iron Loading and Liver Fibrosis in Mice
- PMID: 31127639
- PMCID: PMC6874904
- DOI: 10.1002/hep.30780
Ablation of Hepatocyte Smad1, Smad5, and Smad8 Causes Severe Tissue Iron Loading and Liver Fibrosis in Mice
Abstract
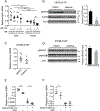
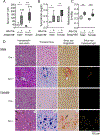
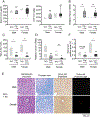
A failure of iron to appropriately regulate liver hepcidin production is central to the pathogenesis of hereditary hemochromatosis. SMAD1/5 transcription factors, activated by bone morphogenetic protein (BMP) signaling, are major regulators of hepcidin production in response to iron; however, the role of SMAD8 and the contribution of SMADs to hepcidin production by other systemic cues remain uncertain. Here, we generated hepatocyte Smad8 single (Smad8fl/fl ;Alb-Cre+ ), Smad1/5/8 triple (Smad158;Alb-Cre+ ), and littermate Smad1/5 double (Smad15;Alb-Cre+ ) knockout mice to investigate the role of SMAD8 in hepcidin and iron homeostasis regulation and liver injury. We found that Smad8;Alb-Cre+ mice exhibited no iron phenotype, whereas Smad158;Alb-Cre+ mice had greater iron overload than Smad15;Alb-Cre+ mice. In contrast to the sexual dimorphism reported for wild-type mice and other hemochromatosis models, hepcidin deficiency and extrahepatic iron loading were similarly severe in Smad15;Alb-Cre+ and Smad158;Alb-Cre+ female compared with male mice. Moreover, epidermal growth factor (EGF) failed to suppress hepcidin in Smad15;Alb-Cre+ hepatocytes. Conversely, hepcidin was still increased by lipopolysaccharide in Smad158;Alb-Cre+ mice, although lower basal hepcidin resulted in lower maximal hepcidin. Finally, unlike most mouse hemochromatosis models, Smad158;Alb-Cre+ developed liver injury and fibrosis at 8 weeks. Liver injury and fibrosis were prevented in Smad158;Alb-Cre+ mice by a low-iron diet and were minimal in iron-loaded Cre- mice. Conclusion: Hepatocyte Smad1/5/8 knockout mice are a model of hemochromatosis that encompasses liver injury and fibrosis seen in human disease. These mice reveal the redundant but critical role of SMAD8 in hepcidin and iron homeostasis regulation, establish a requirement for SMAD1/5/8 in hepcidin regulation by testosterone and EGF but not inflammation, and suggest a pathogenic role for both iron loading and SMAD1/5/8 deficiency in liver injury and fibrosis.
© 2019 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Disclosures
JLB has ownership interest in Ferrumax Pharmaceuticals and has received consulting fees from Keryx Biopharmaceuticals and Disc Medicine. All other authors have nothing to declare.
Figures






Similar articles
-
Smad1/5 is required for erythropoietin-mediated suppression of hepcidin in mice.Blood. 2017 Jul 6;130(1):73-83. doi: 10.1182/blood-2016-12-759423. Epub 2017 Apr 24. Blood. 2017. PMID: 28438754 Free PMC article.
-
Deficiency of the BMP Type I receptor ALK3 partly protects mice from anemia of inflammation.BMC Physiol. 2018 Feb 27;18(1):3. doi: 10.1186/s12899-018-0037-z. BMC Physiol. 2018. PMID: 29482530 Free PMC article.
-
Regulation of iron homeostasis by hepatocyte TfR1 requires HFE and contributes to hepcidin suppression in β-thalassemia.Blood. 2023 Jan 26;141(4):422-432. doi: 10.1182/blood.2022017811. Blood. 2023. PMID: 36322932 Free PMC article.
-
Hepcidin and the BMP-SMAD pathway: An unexpected liaison.Vitam Horm. 2019;110:71-99. doi: 10.1016/bs.vh.2019.01.004. Epub 2019 Feb 10. Vitam Horm. 2019. PMID: 30798817 Review.
-
Integrating positional information at the level of Smad1/5/8.Curr Opin Genet Dev. 2008 Aug;18(4):304-10. doi: 10.1016/j.gde.2008.06.001. Epub 2008 Jul 14. Curr Opin Genet Dev. 2008. PMID: 18590818 Free PMC article. Review.
Cited by
-
MiR-122-5p promotes peritoneal fibrosis in a rat model of peritoneal dialysis by targeting Smad5 to activate Wnt/β-catenin pathway.Ren Fail. 2022 Dec;44(1):191-203. doi: 10.1080/0886022X.2022.2030360. Ren Fail. 2022. PMID: 35170385 Free PMC article.
-
Mechanisms controlling cellular and systemic iron homeostasis.Nat Rev Mol Cell Biol. 2024 Feb;25(2):133-155. doi: 10.1038/s41580-023-00648-1. Epub 2023 Oct 2. Nat Rev Mol Cell Biol. 2024. PMID: 37783783 Review.
-
Unveiling the Impact of BMP9 in Liver Diseases: Insights into Pathogenesis and Therapeutic Potential.Biomolecules. 2024 Aug 15;14(8):1013. doi: 10.3390/biom14081013. Biomolecules. 2024. PMID: 39199400 Free PMC article. Review.
-
Coordination of iron homeostasis by bone morphogenetic proteins: Current understanding and unanswered questions.Dev Dyn. 2022 Jan;251(1):26-46. doi: 10.1002/dvdy.372. Epub 2021 May 25. Dev Dyn. 2022. PMID: 33993583 Free PMC article.
-
LncRNA SPANXA2-OT1 Participates in the Occurrence and Development of EMT in Calcium Oxalate Crystal-Induced Kidney Injury by Adsorbing miR-204 and Up-Regulating Smad5.Front Med (Lausanne). 2021 Sep 27;8:719980. doi: 10.3389/fmed.2021.719980. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34646842 Free PMC article.
References
-
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004;306:2090–2093. - PubMed
-
- Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet 2003;33:21–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

