Down-titration and discontinuation strategies of tumour necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity
- PMID: 31125448
- PMCID: PMC6534285
- DOI: 10.1002/14651858.CD010455.pub3
Down-titration and discontinuation strategies of tumour necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity
Abstract
Background: Anti-tumour necrosis factor (TNF) agents are effective in treating people with rheumatoid arthritis (RA), but are associated with (dose-dependent) adverse effects and high costs. To prevent overtreatment, several trials have assessed the effectiveness of down-titration compared with continuation of the standard dose. This is an update of a Cochrane Review published in 2014.
Objectives: To evaluate the benefits and harms of down-titration (dose reduction, discontinuation, or disease activity-guided dose tapering) of anti-TNF agents on disease activity, functioning, costs, safety, and radiographic damage compared with usual care in people with RA and low disease activity.
Search methods: We searched MEDLINE, Embase, Web of Science and CENTRAL (29 March 2018) and four trial registries (11 April 2018) together with reference checking, citation searching, and contact with study authors to identify additional studies. We screened conference proceedings (American College of Rheumatology and European League Against Rheumatism 2005-2017).
Selection criteria: Randomised controlled trials (RCTs) and controlled clinical trials (CCTs) comparing down-titration (dose reduction, discontinuation, disease activity-guided dose tapering) of anti-TNF agents (adalimumab, certolizumab pegol, etanercept, golimumab, infliximab) to usual care/no down-titration in people with RA and low disease activity.
Data collection and analysis: We used standard Cochrane methodology.
Main results: One previously included trial was excluded retrospectively in this update because it was not an RCT/CCT. We included eight additional trials, for a total of 14 studies (13 RCTs and one CCT, 3315 participants in total) reporting anti-TNF down-titration. Six studies (1148 participants) reported anti-TNF dose reduction compared with anti-TNF continuation. Eight studies (2111 participants) reported anti-TNF discontinuation compared with anti-TNF continuation (three studies assessed both anti-TNF discontinuation and dose reduction), and three studies assessed disease activity-guided anti-TNF dose tapering (365 participants). These studies included data on all anti-TNF agents, but primarily adalimumab and etanercept. Thirteen studies were available in full text, one was available as abstract. We assessed the included studies generally at low to moderate risk of bias; our main concerns were bias due to open-label treatment and unblinded outcome assessment. Clinical heterogeneity between the trials was high. The included studies were performed at clinical centres around the world and included people with early as well as established RA, the majority of whom were female with mean ages between 47 and 60. Study durations ranged from 6 months to 3.5 years.We found that anti-TNF dose reduction leads to little or no difference in mean disease activity score (DAS28) after 26 to 52 weeks (high-certainty evidence, mean difference (MD) 0.06, 95% confidence interval (CI) -0.11 to 0.24, absolute risk difference (ARD) 1%) compared with continuation. Also, anti-TNF dose reduction does not result in an important deterioration in function after 26 to 52 weeks (Health Assessment Questionnaire Disability Index (HAQ-DI)) (high-certainty evidence, MD 0.09, 95% CI 0.00 to 0.19, ARD 3%). Next to this, anti-TNF dose reduction may slightly reduce the proportion of participants switched to another biologic (low-certainty evidence), but probably slightly increases the proportion of participants with minimal radiographic progression after 52 weeks (moderate-certainty evidence, risk ratio (RR) 1.22, 95% CI 0.76 to 1.95, ARD 2% higher). Anti-TNF dose reduction may cause little or no difference in serious adverse events, withdrawals due to adverse events and proportion of participants with persistent remission (low-certainty evidence).Results show that anti-TNF discontinuation probably slightly increases the mean disease activity score (DAS28) after 28 to 52 weeks (moderate-certainty evidence, MD 0.96, 95% CI 0.67 to 1.25, ARD 14%), and that the RR of persistent remission lies between 0.16 and 0.77 (low-certainty evidence). Anti-TNF discontinuation increases the proportion participants with minimal radiographic progression after 52 weeks (high-certainty evidence, RR 1.69, 95% CI 1.10 to 2.59, ARD 7%) and may lead to a slight deterioration in function (HAQ-DI) (low-certainty evidence). It is uncertain whether anti-TNF discontinuation influences the number of serious adverse events (due to very low-certainty evidence) and the number of withdrawals due to adverse events after 28 to 52 weeks probably increases slightly (moderate-certainty evidence, RR 1.46, 95% CI 0.75 to 2.84, ARD 1% higher).Anti-TNF disease activity-guided dose tapering may result in little or no difference in mean disease activity score (DAS28) after 72 to 78 weeks (low-certainty evidence). Furthermore, anti-TNF disease activity-guided dose tapering results in little or no difference in the proportion of participants with persistent remission after 18 months (high-certainty evidence, RR 0.89, 95% CI 0.75 to 1.06, ARD -9%) and may result in little or no difference in switching to another biologic (low-certainty evidence). Anti-TNF disease activity-guided dose tapering may slightly increase proportion of participants with minimal radiographic progression (low-certainty evidence) and probably leads to a slight deterioration of function after 18 months (moderate-certainty evidence, MD 0.2 higher, 0.02 lower to 0.42 higher, ARD 7% higher), It is uncertain whether anti-TNF disease activity-guided dose tapering influences the number of serious adverse events due to very low-certainty evidence.
Authors' conclusions: We found that fixed-dose reduction of anti-TNF, after at least three to 12 months of low disease activity, is comparable to continuation of the standard dose regarding disease activity and function, and may be comparable with regards to the proportion of participants with persistent remission. Discontinuation (also without disease activity-guided adaptation) of anti-TNF is probably inferior to continuation of treatment with respect to disease activity, the proportion of participants with persistent remission, function, and minimal radiographic damage. Disease activity-guided dose tapering of anti-TNF is comparable to continuation of treatment with respect to the proportion of participants with persistent remission and may be comparable regarding disease activity.Caveats of this review are that available data are mainly limited to etanercept and adalimumab, the heterogeneity between studies, and the use of superiority instead of non-inferiority designs.Future research should focus on the anti-TNF agents infliximab and golimumab; assessment of disease activity, function, and radiographic outcomes after longer follow-up; and assessment of long-term safety, cost-effectiveness, and predictors for successful down-titration. Also, use of a validated flare criterion, non-inferiority designs, and disease activity-guided tapering instead of fixed-dose reduction or discontinuation would allow researchers to better interpret study findings and generalise to clinical practice.
Conflict of interest statement
Lise M Verhoef: none known.
Bart(holomeus) JF van den Bemt: none known.
Aatke van der Maas: none known.
Johanna Vriezekolk: none known
Marlies Hulscher: none known.
Frank van den Hoogen: none known.
Wilco Jacobs: none known.
Noortje van Herwaarden: none known.
Alfons A den Broeder: none known.
Figures
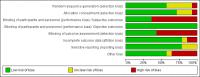
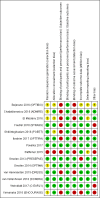

























Update of
-
Down-titration and discontinuation strategies of tumor necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity.Cochrane Database Syst Rev. 2014 Sep 29;(9):CD010455. doi: 10.1002/14651858.CD010455.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 May 24;5:CD010455. doi: 10.1002/14651858.CD010455.pub3. PMID: 25264908 Updated. Review.
Similar articles
-
Down-titration and discontinuation strategies of tumor necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity.Cochrane Database Syst Rev. 2014 Sep 29;(9):CD010455. doi: 10.1002/14651858.CD010455.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 May 24;5:CD010455. doi: 10.1002/14651858.CD010455.pub3. PMID: 25264908 Updated. Review.
-
TNF-alpha inhibitors for ankylosing spondylitis.Cochrane Database Syst Rev. 2015 Apr 18;2015(4):CD005468. doi: 10.1002/14651858.CD005468.pub2. Cochrane Database Syst Rev. 2015. PMID: 25887212 Free PMC article. Review.
-
Biologics or tofacitinib for people with rheumatoid arthritis unsuccessfully treated with biologics: a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 10;3(3):CD012591. doi: 10.1002/14651858.CD012591. Cochrane Database Syst Rev. 2017. PMID: 28282491 Free PMC article. Review.
-
Biologic or tofacitinib monotherapy for rheumatoid arthritis in people with traditional disease-modifying anti-rheumatic drug (DMARD) failure: a Cochrane Systematic Review and network meta-analysis (NMA).Cochrane Database Syst Rev. 2016 Nov 17;11(11):CD012437. doi: 10.1002/14651858.CD012437. Cochrane Database Syst Rev. 2016. PMID: 27855242 Free PMC article. Review.
-
Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2017 May 8;5(5):CD012657. doi: 10.1002/14651858.CD012657. Cochrane Database Syst Rev. 2017. PMID: 28481462 Free PMC article. Review.
Cited by
-
Prevalence and predictors of sustained remission/low disease activity after discontinuation of induction or maintenance treatment with tumor necrosis factor inhibitors in rheumatoid arthritis: a systematic and scoping review.Arthritis Res Ther. 2023 Nov 20;25(1):222. doi: 10.1186/s13075-023-03199-0. Arthritis Res Ther. 2023. PMID: 37986101 Free PMC article.
-
Effect of tapered versus stable treatment with tumour necrosis factor inhibitors on disease flares in patients with rheumatoid arthritis in remission: a randomised, open label, non-inferiority trial.Ann Rheum Dis. 2023 Nov;82(11):1394-1403. doi: 10.1136/ard-2023-224476. Epub 2023 Aug 22. Ann Rheum Dis. 2023. PMID: 37607809 Free PMC article. Clinical Trial.
-
[Treatment algorithm for rheumatoid arthritis : According to the S2e guidelines 2018].Z Rheumatol. 2019 Aug;78(6):529-539. doi: 10.1007/s00393-019-0668-x. Z Rheumatol. 2019. PMID: 31297605 Review. German.
-
Dose reduction of the new generation biologics (IL-17 and IL-23 inhibitors) in psoriasis: study protocol for an international, pragmatic, multicenter, randomized, controlled, non-inferiority study-the BeNeBio study.Trials. 2021 Oct 16;22(1):707. doi: 10.1186/s13063-021-05681-z. Trials. 2021. PMID: 34656148 Free PMC article.
-
Impact of tapering targeted therapies (bDMARDs or JAKis) on the risk of serious infections and adverse events of special interest in patients with rheumatoid arthritis or spondyloarthritis: a systematic analysis of the literature and meta-analysis.Arthritis Res Ther. 2020 Apr 29;22(1):97. doi: 10.1186/s13075-020-02188-x. Arthritis Res Ther. 2020. PMID: 32349791 Free PMC article.
References
References to studies included in this review
Bejerano 2016 (OPTIBIO) {published data only}
-
- Bejerano C, Oreiro N, Fernandez‐Lopez C, Pinto‐Tasende JA, Atanes A, Aspe B, et al. Clinical evaluation usefulness of standardized protocol strategies of dose reduction in patients with rheumatoid arthritis in clinical remission treated with biologic therapies. The OPTIBIO study [abstract]. Arthritis and Rheumatology 2016;68:(Suppl 10).
Chatzidionysiou 2016 (ADMIRE) {published data only}
-
- Chatzidionysiou K, Turesson C, Teleman A, Knight A, Lindqvist E, Larsson P, et al. A multicentre, randomised, controlled, open‐label pilot study on the feasibility of discontinuation of adalimumab in established patients with rheumatoid arthritis in stable clinical remission. RMD Open 2016;2:e000133. [PUBMED: 26819752 ] - PMC - PubMed
-
- Chatzidionysiou K, Turesson C, Teleman A, Knight A, Lindqvist E, Larsson P, et al. Multicenter, randomised, controlled, open‐label pilot study of the feasibility of discontinuation of adalimumab in rheumatoid arthritis patients is stable clinical remission. Arthritis and Rheumatism 2012;64(Suppl 10):776. - PMC - PubMed
El Miedany 2016 {published data only}
-
- Miedany Y, Gaafary M, Ahmed I, Bahlas S, Hegazi M, Nasr A. Optimizing therapy in inflammatory arthritis: prediction of relapse after tapering or stopping treatment for rheumatoid arthritis patients achieving clinical and radiological remission. Clinical Rheumatology 2016;35:2915‐23. [PUBMED: 27658417] - PubMed
Fautrel 2016 (STRASS) {published data only}
-
- Fautrel B, Gandjbakhch F, Foltz T, Pham T, Morel J, Alfaiate T, et al. Targeting the lowest efficacious dose for rheumatoid arthritis patients in remission: clinical and structural impact of a step‐down strategy trial based on progressive spacing of TNF‐blocker injections (STRASS trial). Annals of Rheumatic Disease 2013;72(Suppl 3):72.
-
- Fautrel B, Pham T, Alfaiate T, Gandjbakhch F, Foltz V, Morel J, et al. Step‐down strategy of spacing TNF‐blocker injections for established rheumatoid arthritis in remission: results of the multicentre non‐inferiority randomised open‐label controlled trial (STRASS: Spacing of TNF‐blocker injections in Rheumatoid ArthritiS Study). Annals of the Rheumatic Diseases 2016;75(1):59‐67. [PUBMED: 26103979 ] - PubMed
-
- Fautrel B, Pham T, Tubach F, Alfaiate T, Morel J, Dernis E, et al. Tapering TNF‐blockers in established rheumatoid arthritis patients in DAS28 remission: results of a DAS28‐driven step‐down strategy randomized controlled trial. Arthritis and Rheumatism 2012;64(12):4169‐70.
-
- Vanier A, Mariette X, Tubach F, Fautrel B. Cost‐Effectiveness of TNF‐Blocker Injection Spacing for Patients with Established Rheumatoid Arthritis in Remission: An Economic Evaluation from the Spacing of TNF‐Blocker Injections in Rheumatoid Arthritis Trial.. Value in Health 2017;20(4):577‐585. - PubMed
Ghiti Moghadam 2016 (POEET) {published data only}
-
- Ghiti Moghadam M, Vonkeman HE, Klooster PM, Tekstra J, Schaardenburg D, Starmans‐Kool M, et al. Stopping tumor necrosis factor‐inhibitors in patients with established rheumatoid arthritis in remission or stable low disease activity: a pragmatic randomized multicenter open‐label controlled trial. Arthritis and Rheumatology 2016;68(8):1810‐7. - PubMed
Ibrahim 2017 (OPTTIRA) {published data only}
-
- Galloway JB, Kingsley G, Ma M, Lorente‐Canovas B, Cope A, Ibrahim F, et al. Optimising treatment with TNF inhibitors in rheumatoid arthritis with different dose tapering strategies: the OPTTIRA trial. Annals of the Rheumatic Diseases 2015;74 (Suppl 2):706.
-
- Ibrahim F, Lorente‐Cánovas B, Doré CJ, Bosworth A, Ma MH, Galloway JB, et al. Optimizing treatment with tumour necrosis factor inhibitors in rheumatoid arthritis ‐ a proof of principle and exploratory trial: is dose tapering practical in good responders?. Rheumatology 2017;56:2004‐14. [PUBMED: 28968858] - PMC - PubMed
Pavelka 2017 {published data only}
-
- NCT01578850. Study conducted in subjects with rheumatoid arthritis who have moderate to severe disease activity despite methotrexate therapy with or without other non biologic disease modifying antirheumatic drugs (DMARDs) for at least 12 weeks prior to screening. https://clinicaltrials.gov/ct2/show/results/NCT01578850 First posted April 17, 2012.
-
- Pavelka K, Akkoç N, Al‐Maini M, Zerbini CAF, Karateev DE, Nasonov EL, et al. Maintenance of remission with combination etanercept–DMARD therapy versus DMARDs alone in active rheumatoid arthritis: results of an international treat‑to‑target study conducted in regions with limited biologic access. Rheumatology International 2017;37:1469‐79. [PUBMED: 28597306] - PubMed
Raffeiner 2015 {published data only}
-
- Botsios C, Furlan A, Ostuni P, Sfriso P, Todesco S, Punzi L. Effects of low‐dose etanercept in maintaining DAS‐remission previously achieved with standard‐dose in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 2007;66(Suppl II):54.
-
- Raffeiner B, Botsios C, Ometto F, Bernardi L, Stramare R, Todesco S, et al. Effects of half dose etanercept (25 mg once a week) on clinical remission and radiographic progression in patients with rheumatoid arthritis in clinical remission achieved with standard dose. Clinical and Experimental Rheumatology 2015;33:63‐8. [PUBMED: 25535985] - PubMed
Smolen 2013 (PRESERVE) {published data only}
-
- Smolen JS, Nash P, Durez P, Hall S, Ilivanova E, Irazoque‐Palazuelos F, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013;381:918‐29. - PubMed
Smolen 2014 (OPTIMA) {published data only}
-
- Emery P, Smolen JS, Kavanaugh A, Vollenhoven R, Pavelka K, Durez P, et al. Maintenance of biologic‐free disease control in early rheumatoid arthritis patients after induction of low disease activity with adalimumab plus methotrexate. Annals of the Rheumatic Diseases 2011;70 (Suppl 3):262.
-
- Smolen JS, Emery P, Fleischmann R, Vollenhoven R, Florentius S, Santra S, et al. Biologic free disease control (BFDC) in early RA: predictors of successful withdrawal of adalimumab (ADA) after achieving stable low disease activity with ADA plus methotrexate (MTX) ‐ data from the OPTIMA study. Annals of the Rheumatic Diseases 2012;71 (Suppl 3):516.
-
- Smolen JS, Emery P, Fleischmann R, Vollenhoven RF, Pavelka K, Durez P, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 2014;383:321‐32. [PUBMED: 24168956] - PubMed
van Herwaarden 2015 (DRESS) {published data only}
-
- Kievit W, Herwaarden N, Hoogen FHJ, Vollenhoven RF, Bijlsma JWJ, Bemt BJF, et al. Disease activity‐guided dose optimisation of adalimumab and etanercept is a cost‐effective strategy compared with non‐tapering tight control rheumatoid arthritis care: analyses of the DRESS study. Annals of the Rheumatic Diseases 2016;75(11):1939‐1944. [PUBMED: 26764260] - PubMed
-
- Herwaarden N, Maas A, Minten MJM, Hoogen FHJ, Kievit W, Vollenhoven RF, et al. Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non‐inferiority trial. BMJ 2015;350:h1389. [PUBMED: 25858265] - PMC - PubMed
van Vollenhoven 2016 (DOSERA) {published data only}
-
- Vollenhoven R, Østergaard M, Leirisalo‐Repo M, Uhlig T, Jansson M, Klackenberg A, et al. Rheumatoid arthritis patients with stable low disease activity on methotrexate plus etanercept, continuation of etanercept 50 mg weekly or 25 mg weekly are both clinically superior to discontinuation: results from a randomized, 3‐armed, double‐blind clinical trial. Arthritis and Rheumatism 2012;64(12):4171.
Weinblatt 2017 (C‐EARLY) {published data only}
-
- Weinblatt ME, Bingham CO 3rd, Burmester G, Bykerk VP, Furst DE, Mariette X, et al. A phase III study evaluating continuation, tapering, and withdrawal of certolizumab pegol after one year of therapy in patients with early rheumatoid arthritis. Arthritis & Rheumatology 2017;69:1937‐48. [PUBMED: 28666080] - PMC - PubMed
Yamanaka 2016 (ENCOURAGE) {published data only}
-
- Yamanaka H, Nagaoka S, Lee S, Bae S, Kasama T, Kobayashi H, et al. Discontinuation of etanercept after achievement of sustained remission in patients with rheumatoid arthritis who initially had moderate disease activity ‐ results from the ENCOURAGE study, a prospective, international, multicenter randomized study. Modern Rheumatology 2016;26(5):651‐61. [PUBMED: 26698929] - PubMed
References to studies excluded from this review
Aletaha 2010 {published data only}
-
- Aletaha D. Challenging the course of RA: time for drug‐free remission?. Nature Reviews Rheumatology 2010;6:442‐3. - PubMed
Awan 2011 {published data only}
-
- Awan S, Bannon C, O'Sullivan, Duffy M, Murphy E, Barry M. Sustained remission at 6 months, despite reduction in anti‐TNF dosing frequency in inflammatory arthritis. Irish Journal of Medical Science 2011;181(Suppl 2):54.
Bejarano 2010 {published data only}
-
- Bejarano V, Conoghan P, Quinn MA, Saleem B, Emery P. Benefits 8 years after a remission induction regime with an infliximab and methotrexate combination in early rheumatoid arthritis. Rheumatology 2010;49:1971‐4. - PubMed
CADTH Report 2014 {published data only}
-
- Unknown. Long‐term sustained clinical remission after stopping first‐line anti‐TNF agents in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis: clinical effectiveness. Canadian Agency for Drugs and Technologies in Health 2014.
Detert 2013 (HIT‐HARD) {published data only}
-
- Detert J, Bastion H, Listing J, Weiss A, Wassenberg S, Liebhaber A, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD‐naïve patients with early rheumatoid arthritis: HIT HARD, an investigator‐initiated study. Annals of the Rheumatic Diseases 2013;72:844‐50. - PubMed
Emery 2013 (PRIZE) {published data only}
-
- Emery P, Hammoudeh M, FitzGerald O, Combe B, Martin Mola E, Bukowski J, et al. Assessing maintenance of remission with reduced dose etanercept plus methotrexate, methotrexate alone or placebo in patients with early rheumatoid arthritis who achieved remission with etanercept and methotrexate: the PRIZE study. Annals of the Rheumatic Diseases 2013;72 (Suppl 3):399.
Greenberg 2014 {published data only}
-
- Greenberg JD, Shan Y, Reed GW, Bitman B, Collier D. Comparison of switching to reduced dose vs continuation of standard dose etanercept for rheumatoid arthritis patients in the CORRONA registry. Annals of the Rheumatic Diseases 2014;73 (Suppl 2):241.
Haraoui 2014 {published data only}
-
- Haraoui B, Bykerk VP, Vollenhoven R, Longueville M, Luijtens K, Ralston P, et al. Analysis of pooled data from two randomized controlled trials and their open‐label extensions: long‐term safety in rheumatoid arthritis before and after certolizumab pegol dose increase/decrease. Arthritis & Rheumatology 2014;66:S199.
Harigai 2012 (BRIGHT) {published data only}
-
- Harigai M, Takeuchi T, Tanaka, Y, Matsubara T, Yamanaka H, Miyasaka N. Discontinuation of adalimumab treatment in rheumatoid arthritis patients after achieving low disease activity. Modern Rheumatology 2012;22:814‐22. - PubMed
Haschka 2016 (RETRO) {published data only}
-
- Haschka J, Englbrecht M, Hueber AJ, Manger B, Kleyer A, Reiser M, et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic therapy: interim results from the prospective randomised controlled RETRO study. Annals of the Rheumatic Diseases 2016;75:45‐51. [PUBMED: 25660991] - PubMed
Heimans 2016 (IMPROVED) {published data only}
-
- Heimans L, Akdemir G, Wevers‐de Boer KVC, Goekoop‐Ruiterman YP, Molenaar ET, Groenendael JHLM, et al. Two‐year results of disease activity score (DAS)‐remission‐steered treatment strategies aiming at drug‐free remission in early arthritis patients (the IMPROVED‐study). Arthritis Research and Therapy 2016;28:23. [26794605] - PMC - PubMed
Ichikawa 2007 {published data only}
-
- Ichikawa N, Yamanaka H. Maintenance therapy for rheumatoid arthritis after remission following successful treatment using biologics. Nihon Rinsho 2007;65(7):1293‐8. - PubMed
Keystone 2003 {published data only}
-
- Keystone EC, Haraoui B, Bykerk VP. Role of infliximab in the treatment of early rheumatoid arthritis. Clinical and Experimental Rheumatology 2003;21 (Suppl 31):S200‐2. - PubMed
Klarenbeek 2011 {published data only}
-
- Klarenbeek NB, Kooij SM, Güler‐Yüksel M, Groenendael JHLM, Han KH, Kerstens PJSM, et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Annals of the Rheumatic Diseases 2011;70:315‐9. - PubMed
Kobelt 2011 {published data only}
-
- Kobelt G, Lekander I, Lang A, Raffeiner B, Botsios C, Geborek P. Cost‐effectiveness of etanercept treatment in early active rheumatoid arthritis followed by dose adjustment. International Journal of Technology Assessment in Health Care 2011;27(3):193‐200. - PubMed
Kobelt 2014 {published data only}
-
- Kobelt G. Treating to target with etanercept in rheumatoid arthritis: cost‐effectiveness of dose reductions when remission is achieved. Value in Health 2014;17:537‐44. [PUBMED: 25128046] - PubMed
Oba 2017 (RRRR study) {published data only}
-
- Oba K, Norie N, Sato N, Saito K, Takeuchi T, Mimori T, et al. Remission induction by Raising the dose of Remicade in RA (RRRR) study: rationale and study protocol for a randomized controlled trial comparing for sustained clinical remission after discontinuation of infliximab in patients with rheumatoid arthritis. Contemporary Clinical Trials Communications 2017;8:49‐54. [PUBMED: 29696196 ] - PMC - PubMed
Quinn 2005 {published data only}
-
- Quinn MA, Conaghan PG, O'Connor PJ, Karim Z, Greenstein A, Brown A, et al. Very early treatment with infliximab in addition to methotrexate in early, poor‐prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve‐month randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism 2005;52(1):27‐35. - PubMed
Rakieh 2013 {published data only}
-
- Rakieh C, Saleem B, Takase K, Nam JL, Keen H, Wakefield RJ, et al. Long term outcomes of stopping tumour necrosis factor inhibitors (TNFi) in patients with established rheumatoid arthritis (RA) who are in sustained remission: is it worth the risk?. Annals of the Rheumatic Diseases 2013;72 (Suppl 3):208‐9.
Ramírez‐Herráiz 2013 {published data only}
-
- Ramírez‐Herráiz E, Escudero‐Vilaplana V, Alañón‐Plaza E, Trovato‐López N, Herranz‐Alonso A, Morell‐Baladrón A, et al. Efficiency of adalimumab, etanercept and infliximab in rheumatoid arthritis patients: dosing patterns and effectiveness in daily clinical practice. Clinical and Experimental Rheumatology 2013;31(4):559‐65. - PubMed
Seddighzadeh 2014 (NORD‐STAR) {published data only}
-
- Seddighzadeh M. NORD‐STAR: a multicentre, randomized, open‐label, blinded‐assessor, phase 4 study in patients with early rheumatoid arthritis. Scandinavian Journal of Rheumatology 2014;43 (Suppl 127):31‐2.
Smolen 2012 (CERTAIN) {published data only}
-
- Smolen JS, Emery P, Ferraccioli G, Samborski W, Berenbaum F, Davies O, et al. Maintenance of remission in rheumatoid arthritis patients with low‐moderate disease activity following withdrawal of certolizumab pegol treatment: week 52 results from the CERTAIN study. Annals of the Rheumatic Diseases 2012;71 (Suppl 3):25.
Tada 2012 (PRECEPT) {published data only}
-
- Tada M, Koiki T, Okano T, Sugioka Y, Wakitani S, Fukushima K, et al. Comparison of joint destruction between standard‐ and low‐dose etanercept in rheumatoid arthritis from the Prevention of Cartilage Destruction by Etanercept (PRECEPT) study. Rheumatology 2012;51:2164‐9. - PubMed
Tanaka 2013 (HONOR) {published data only}
Tanaka 2014 (HOPEFUL‐2) {published data only}
-
- Tanaka Y, Yamanaka H, Ishiguro N, Miyasaka N, Kawana K, Hiramatsu K, et al. Attainment of low disease activity is predictive of maintenance of disease control upon adalimumab discontinuation for two years following combination therapy in Japanese patients with early rheumatoid arthritis. Arthritis & Rheumatology 2014;66:S1059. [Abstract number: 2427]
van den Broek 2011 {published data only}
-
- Broek M, Klarenbeek NB, Dirven L, Schaardenburg D, Hulsmans HMJ, Kerstens PJSM, et al. Discontinuation of infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score‐steered therapy: subanalysis of the BeSt study. Annals of the Rheumatic Diseases 2011;70:1389‐94. - PubMed
van der Kooij 2009 {published data only}
-
- Kooij SM, Goekoop‐Ruiterman YPM, Vries‐Bouwstra JK, Guler‐Yuksel M, Zwinderman AH, Kerstens PJSM, et al. Drug‐free remission, functioning and radiographic damage after 4 years of response‐driven treatment in patients with recent‐onset rheumatoid arthritis. Annals of the Rheumatic Diseases 2009;68:914‐21. - PubMed
Villeneuve 2012 {published data only}
-
- Villeneuve E, Nam E, Hensor JL, Wakefield E, Conoghan RJ, Green PG, et al. Preliminary results of a multicentre randomised controlled trial of etanercept and methotrexate to induce remission in patients with newly diagnosed inflammatory arthritis. Arthritis and Rheumatism 2011;63 (Suppl 10):2465.
Wiland 2016 (PRIZE) {published data only}
-
- Wiland P, Dudler J, Veale D, Tahir H, Pedersen R, Bukowski J, et al. The effect of reduced or withdrawn etanercept‐methotrexate therapy on patient‐reported outcomes in patients with early rheumatoid arthritis. Journal of Rheumatology 2016;43:1268‐77. [PUBMED: 27252426] - PubMed
References to ongoing studies
2012‐004631‐22 {published data only}
-
- 2012‐004631‐22. TapERA: Maintaining remission in RA while tapering Etanercept.. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2012‐004631‐22/BE Date of first entry 11‐12‐2012.
2017‐001970‐41 {published data only}
-
- 2017‐001970‐41. The BIODOPT trial (BIOlogical Dose OPTimisation). https://www.clinicaltrialsregister.eu/ctr‐search/trial/2017‐001970‐41/DK Date first received 15‐09‐2017.
NCT01793519 {published data only}
-
- NCT01793519. Stopping TNF Alpha Inhibitors in Rheumatoid Arthritis (STARA). https://clinicaltrials.gov/ct2/show/NCT01793519 First posted on February 15, 2013.
NCT01881308 {published data only}
-
- NCT01881308. Assessing Withdrawal of Disease‐Modifying Antirheumatic Drugs in Rheumatoid Arthritis (ARCTIC REWIND). https://clinicaltrials.gov/ct2/show/NCT01881308 First posted on June 19, 2013.
NCT02198651 {published data only}
-
- NCT02198651. A Phase 4 Trial Assessing the ImPact of Residual Inflammation Detected Via Imaging TEchniques, Drug Levels and Patient Characteristics on the Outcome of Dose TaperIng of Adalimumab in Clinical Remission Rheumatoid ArThritis (RA) Subjects (PREDICTRA). https://clinicaltrials.gov/ct2/show/NCT02198651 First posted on July 24, 2014. - PMC - PubMed
NCT02373813 {published data only}
-
- NCT02373813. Study of Etanercept Monotherapy vs Methotrexate Monotherapy for Maintenance of Rheumatoid Arthritis Remission. https://clinicaltrials.gov/ct2/show/NCT02373813 First posted on February 27, 2015.
NTR3903 {published data only}
-
- NTR3903. Dose‐to‐target of etanercept treatment in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis.. https://www.trialregister.nl/trial/3705 Register date 2013‐03‐14.
Additional references
Aletaha 2005
-
- Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clinical and Experimental Rheumatology 2005;23(Suppl 39):S100‐8. - PubMed
Aletaha 2010 RA criteria
-
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis and Rheumatism 2010;62(9):2569‐81. - PubMed
Arnett 1988
-
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism 1988;31(3):315‐24. - PubMed
Bartelds 2007
Blumenauer 2002
Blumenauer 2003
Bongartz 2006
-
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti‐TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta‐analysis of rare harmful effects in randomised controlled trials. JAMA 2006;295(19):2275‐85. - PubMed
Brocq 2009
-
- Brocq O, Millasseau E, Albert C, Grisot C, Flory P, Roux CH, et al. Effect of discontinuing TNFa antagonist therapy in patients with remission of rheumatoid arthritis. Joint Bone Spine 2009;76(4):350‐5. - PubMed
den Broeder 2002
-
- Broeder AA, Creemers MCW, Gestel AM, Riel PLCM. Dose titration using the Disease Activity Score (DAS28) in rheumatoid arthritis patients treated with anti‐TNF‐α. Rheumatology 2002;41(6):638‐42. - PubMed
den Broeder 2010
-
- Broeder AA, Maas A, Bemt BJF. Dose de‐escalation strategies and role of therapeutic drug monitoring of biologics in RA. Rheumatology 2010;49(10):1801‐3. - PubMed
Doherty 2009
-
- Doherty M, Dieppe P. The ‘placebo’ response in osteoarthritis and its implications for clinical practice. Osteoarthritis Cartilage 2009;17(10):1255‐62. - PubMed
Fautrel 2015
-
- Fautrel B, Broeder AA. De‐intensifying treatment in established rheumatoid arthritis (RA): why, how, when and in whom can DMARDs be tapered?. Best Practice & Research: Clinical Rheumatology 2015;29(4‐5):550‐65. [PUBMED: 26697766] - PubMed
Felson 2011
Fransen 2005
-
- Fransen J, Riel PLCM. The Disease Activity Score and the EULAR response criteria. Clinical Experimental Rheumatology 2005;23(5 Suppl 39):S93‐9. - PubMed
Galvao 2016
-
- Galvao TF, Zimmerman IR, Mota LMH, Silva MT, Pereira MG. Withdrawal of biologic agents in rheumatoid arthritis: a systematic review and meta‐analysis. Clinical Rheumatology 2016;35:1659‐68. - PubMed
Genovese 2002
-
- Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two‐year radiographic and clinical outcomes. Arthritis & Rheumatology 2002;46(6):1443‐50. [PUBMED: 12115173] - PubMed
GRADEpro 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed on 28 June 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kavanaugh 2012
-
- Kavanaugh A, Emery P, Vollenhoven R, Cifaldi M, Shaw J, Chen N, et al. Effect of adalimumab discontinuation on patient‐reported outcomes and work productivity in early rheumatoid arthritis patients who achieved low disease activity following 26 weeks of treatment: data from the OPTIMA study. Annals of the Rheumatic Diseases 2012;71 (Suppl 3):663.
Klareskog 2011
-
- Klareskog L, Gaubitz M, Rodríguez‐Valverde V, Malaise M, Dougados M, Wajdula J. Assessment of long‐term safety and efficacy of etanercept in a 5‐year extension study in patients with rheumatoid arthritis. Clinical Experimental Rheumatology 2011;29(2):238‐47. - PubMed
Kuijper 2015
-
- Kuijper TM, Lamers‐Karnebeek FBG, Jacobs JWG, Hazes JMW, Luime JJ. Flare rate in patients with rheumatoid arthritis in low disease activity or remission when tapering or stopping synthetic or biologic DMARD: a systematic review. Journal of Rheumatology 2015;42(11):2012‐22. [PUBMED: 26428204] - PubMed
Larsen 1973
-
- Larsen A. A radiological method for grading the severity of rheumatoid arthritis. Scandinavian Journal of Rheumatology 1973;4(4):225‐33. - PubMed
Maini 1998
-
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor alpha monoclonal antibody combined with low‐dose weekly methotrexate in rheumatoid arthritis. Arthritis & Rheumatology 1998;41(9):1552‐63. [PUBMED: 9751087] - PubMed
Navarro‐Millán 2013
Navarro‐Sarabia 2005
Nawata 2008
-
- Nawata M, Saito K, Nakayamada S, Tanaka Y. Discontinuation of infliximab in rheumatoid arthritis patients in clinical remission. Modern Rheumatology 2008;18:460‐4. - PubMed
Prevoo 1995
-
- Prevoo ML, 't Hof MA, Kuper HH, Leeuwen MA, Putte LB, Riel PL. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis and Rheumatism 1995;38(1):44‐8. - PubMed
Ramiro 2017
-
- Ramiro S, Sepriano A, Chatzidionysiou K, Nam JL, Smolen JS, Heijden D, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Annals of the Rheumatic Diseases 2017;76(6):1101‐1136. [PUBMED: 28298374] - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruiz Garcia 2014
Saleem 2010
-
- Saleem B, Keen H, Goeb V, Parmar R, Nizam S, Hensor EMA. Patients with RA in remission on TNF blockers: when and in whom can TNF blocker therapy be stopped?. Annals of the Rheumatic Diseases 2010;69:1636‐42. - PubMed
Schipper 2010
-
- Schipper LG, Hulst LTC, Grol R, Riel PLCM, Hulscher MEJL, Fransen J. Meta‐analysis of tight control strategies in rheumatoid arthritis: protocolized treatment has additional value with respect to the clinical outcome. Rheumatology 2010;49:2154‐64. [PUBMED: 20671022] - PubMed
Sharp 1971
-
- Sharp JT, Lidsky MD, Collins LC, Moreland J. Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis and Rheumatism 1971;14(6):706‐20. - PubMed
Singh 2009
Singh 2010
Singh 2011
Singh 2016
-
- Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care & Research 2016;68(1):1‐25. [PUBMED: 26545825] - PubMed
Smolen 2003
-
- Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42(2):244‐57. - PubMed
Smolen 2017
-
- Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2016 update. Annals of the Rheumatic Diseases 2017;76(6):960‐977. [PUBMED: 28264816] - PubMed
St Clair 2004
-
- Clair EW, Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Active‐Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset Study Group. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis and Rheumatism 2004;50(11):3432‐43. - PubMed
Tanaka 2010
Tanaka 2012
-
- Tanaka Y, Hirata S, Fukuyo S, Nawata M, Kubo S, Yamaoka K, et al. Discontinuation of adalimumab without functional and radiographic damage progression after achieving sustained remission in patients with rheumatoid arthritis (the HONOR study): 1‐year results. Arthritis and Rheumatism 2012;64(Suppl 10):771.
Tweehuysen 2017
van den Bemt 2008
-
- Bemt BJF, Broeder AA, Snijders GF, Hekster YA, Riel PLCM, Benraad B, et al. Sustained effect after lowering high‐dose infliximab in patients with rheumatoid arthritis: a prospective dose titration study. Annals of the Rheumatic Diseases 2008;67(12):1697‐701. - PubMed
van der Bijl 2007
-
- Bijl AE, Goekoop‐Ruiterman YPM, Vries‐Bouwstra JK, Wolde S, Han KH, Krugten MV, et al. Infliximab and methotrexate as induction therapy in patients with early rheumatoid arthritis. Arthritis and Rheumatism 2007;56(7):2129‐34. - PubMed
van der Heijde 1990
van der Heijde 2000
-
- Heijde D. How to read radiographs according to the Sharp/van der Heijde method. Journal of Rheumatology 2000;27(1):261‐3. - PubMed
van der Maas 2012
-
- Maas A, Kievit W, Bemt BJF, Hoogen FHJ, Riel PL, Broeder AA. Down‐titration and discontinuation of infliximab in rheumatoid arthritis patients with stable low disease activity and stable treatment: an observational cohort study. Annals of the Rheumatic Diseases 2012;71(11):1849‐54. - PubMed
van der Maas 2013 Flare
-
- Maas A, Lie E, Christensen R, Choy E, Man Y, Riel P, et al. Construct and criterion validity of several proposed DAS28‐based rheumatoid arthritis flare criteria: an OMERACT cohort validation study. Annals of the Rheumatic Diseases 2013;72:1800‐5. - PubMed
van Vollenhoven 2004
van Vollenhoven 2009
-
- Vollenhoven RF. How to dose infliximab in rheumatoid arthritis: new data on a serious issue. Annals of the Rheumatic Diseases 2009;48(11):1237‐9. - PubMed
Verhoef 2017
Weinblatt 2003
-
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis & Rheumatology 2003;48(3):855. [PUBMED: 12528101] - PubMed
Wolbink 2006
-
- Wolbink GJ, Vis M, Lems W, Voskuyl AE, Groot E, Nurmohamed MT. Development of anti‐infliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis and Rheumatism 2006;54(3):711‐5. - PubMed
References to other published versions of this review
van Herwaarden 2014
-
- Herwaarden N, Broeder AA, Jacobs W, Maas A, Bijlsma JWJ, Vollenhoven RF, et al. Down‐titration and discontinuation strategies of tumor necrosis factor–blocking agents for rheumatoid arthritis in patients with low disease activity. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD010455.pub2; PUBMED: 25264908] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

