Prenatal inflammation impairs intestinal microvascular development through a TNF-dependent mechanism and predisposes newborn mice to necrotizing enterocolitis
- PMID: 31125264
- PMCID: PMC6689733
- DOI: 10.1152/ajpgi.00332.2018
Prenatal inflammation impairs intestinal microvascular development through a TNF-dependent mechanism and predisposes newborn mice to necrotizing enterocolitis
Abstract
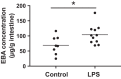
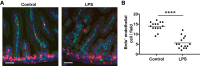
Prenatal inflammation is a risk factor for necrotizing enterocolitis (NEC), and it increases intestinal injury in a rat NEC model. We previously showed that maldevelopment of the intestinal microvasculature and lack of vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) signaling play a role in experimental NEC. However, whether prenatal inflammation affects the intestinal microvasculature remains unknown. In this study, mouse dams were injected intraperitoneally with lipopolysaccharide (LPS) or saline at embryonic day 17. Neonatal intestinal microvasculature density, endothelial cell proliferation, and intestinal VEGF-A and VEGFR2 proteins were assessed in vivo. Maternal and fetal serum TNF concentrations were measured by ELISA. The impact of TNF on the neonatal intestinal microvasculature was examined in vitro and in vivo, and we determined whether prenatal LPS injection exacerbates experimental NEC via TNF. Here we found that prenatal LPS injection significantly decreased intestinal microvascular density, endothelial cell proliferation, and VEGF and VEGFR2 protein expression in neonatal mice. Prenatal LPS injection increased maternal and fetal serum levels of TNF. TNF decreased VEGFR2 protein in vitro in neonatal endothelial cells. Postnatal TNF administration in vivo decreased intestinal microvasculature density, endothelial cell proliferation, and VEGF and VEGFR2 protein expression and increased the incidence of severe NEC. These effects were ameliorated by stabilizing hypoxia-inducible factor-1α, the master regulator of VEGF. Furthermore, prenatal LPS injection significantly increased the incidence of severe NEC in our model, and the effect was dependent on endogenous TNF. Our study suggests that prenatal inflammation increases the susceptibility to NEC, downregulates intestinal VEGFR2 signaling, and affects perinatal intestinal microvascular development via a TNF mechanism. NEW & NOTEWORTHY This report provides new evidence that maternal inflammation decreases neonatal intestinal VEGF receptor 2 signaling and endothelial cell proliferation, impairs intestinal microvascular development, and predisposes neonatal mouse pups to necrotizing enterocolitis (NEC) through inflammatory cytokines such as TNF. Our data suggest that alteration of intestinal microvascular development may be a key mechanism by which premature infants exposed to prenatal inflammation are at risk for NEC and preserving the VEGF/VEGF receptor 2 signaling pathway may help prevent NEC development.
Keywords: endothelial cells; inflammation; intestinal microvasculature; necrotizing enterocolitis; neonate.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





Similar articles
-
Lack of VEGFR2 signaling causes maldevelopment of the intestinal microvasculature and facilitates necrotizing enterocolitis in neonatal mice.Am J Physiol Gastrointest Liver Physiol. 2016 May 1;310(9):G716-25. doi: 10.1152/ajpgi.00273.2015. Epub 2016 Feb 25. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26950855 Free PMC article.
-
Dimethyloxalylglycine preserves the intestinal microvasculature and protects against intestinal injury in a neonatal mouse NEC model: role of VEGF signaling.Pediatr Res. 2018 Feb;83(2):545-553. doi: 10.1038/pr.2017.219. Epub 2017 Oct 25. Pediatr Res. 2018. PMID: 29068435 Free PMC article.
-
Recombinant IGF-1/BP3 protects against intestinal injury in a neonatal mouse NEC model.Pediatr Res. 2024 Jun;95(7):1803-1811. doi: 10.1038/s41390-024-03069-8. Epub 2024 Feb 28. Pediatr Res. 2024. PMID: 38418592
-
Intestinal microcirculation and necrotizing enterocolitis: The vascular endothelial growth factor system.Semin Fetal Neonatal Med. 2018 Dec;23(6):411-415. doi: 10.1016/j.siny.2018.08.008. Epub 2018 Sep 6. Semin Fetal Neonatal Med. 2018. PMID: 30213591 Review.
-
Toll-Like Receptor-Mediated Intestinal Inflammatory Imbalance in the Pathogenesis of Necrotizing Enterocolitis.Cell Mol Gastroenterol Hepatol. 2018 Apr 6;6(2):229-238.e1. doi: 10.1016/j.jcmgh.2018.04.001. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30105286 Free PMC article. Review.
Cited by
-
Impact of fetal inflammatory response on the severity of necrotizing enterocolitis in preterm infants.Pediatr Res. 2024 Apr;95(5):1308-1315. doi: 10.1038/s41390-023-02942-2. Epub 2023 Dec 8. Pediatr Res. 2024. PMID: 38066247
-
Human Milk Growth Factors and Their Role in NEC Prevention: A Narrative Review.Nutrients. 2021 Oct 23;13(11):3751. doi: 10.3390/nu13113751. Nutrients. 2021. PMID: 34836007 Free PMC article. Review.
-
Macrophage-derived IGF-1 protects the neonatal intestine against necrotizing enterocolitis by promoting microvascular development.Commun Biol. 2022 Apr 6;5(1):320. doi: 10.1038/s42003-022-03252-9. Commun Biol. 2022. PMID: 35388142 Free PMC article.
-
Mechanisms and functions of intestinal vascular specialization.J Exp Med. 2024 Jan 1;221(1):e20222008. doi: 10.1084/jem.20222008. Epub 2023 Dec 5. J Exp Med. 2024. PMID: 38051275 Free PMC article. Review.
-
The fetal inflammatory response syndrome: the origins of a concept, pathophysiology, diagnosis, and obstetrical implications.Semin Fetal Neonatal Med. 2020 Aug;25(4):101146. doi: 10.1016/j.siny.2020.101146. Epub 2020 Oct 23. Semin Fetal Neonatal Med. 2020. PMID: 33164775 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

