A genetically encoded toolkit of functionalized nanobodies against fluorescent proteins for visualizing and manipulating intracellular signalling
- PMID: 31122229
- PMCID: PMC6533734
- DOI: 10.1186/s12915-019-0662-4
A genetically encoded toolkit of functionalized nanobodies against fluorescent proteins for visualizing and manipulating intracellular signalling
Abstract
Background: Intrabodies enable targeting of proteins in live cells, but generating specific intrabodies against the thousands of proteins in a proteome poses a challenge. We leverage the widespread availability of fluorescently labelled proteins to visualize and manipulate intracellular signalling pathways in live cells by using nanobodies targeting fluorescent protein tags.
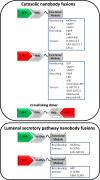
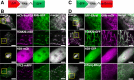
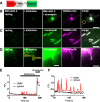
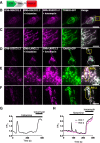
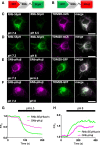
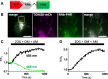
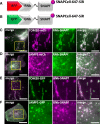
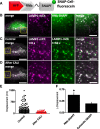
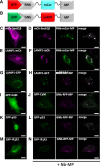
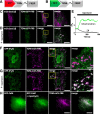
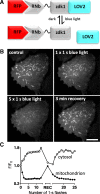
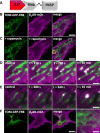
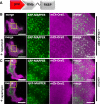
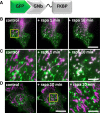
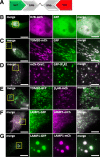
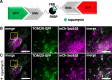
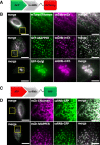
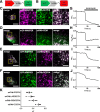
Results: We generated a toolkit of plasmids encoding nanobodies against red and green fluorescent proteins (RFP and GFP variants), fused to functional modules. These include fluorescent sensors for visualization of Ca2+, H+ and ATP/ADP dynamics; oligomerising or heterodimerising modules that allow recruitment or sequestration of proteins and identification of membrane contact sites between organelles; SNAP tags that allow labelling with fluorescent dyes and targeted chromophore-assisted light inactivation; and nanobodies targeted to lumenal sub-compartments of the secretory pathway. We also developed two methods for crosslinking tagged proteins: a dimeric nanobody, and RFP-targeting and GFP-targeting nanobodies fused to complementary hetero-dimerizing domains. We show various applications of the toolkit and demonstrate, for example, that IP3 receptors deliver Ca2+ to the outer membrane of only a subset of mitochondria and that only one or two sites on a mitochondrion form membrane contacts with the plasma membrane.
Conclusions: This toolkit greatly expands the utility of intrabodies and will enable a range of approaches for studying and manipulating cell signalling in live cells.
Keywords: Cell signalling; Endoplasmic reticulum; Fluorescence microscopy; Fluorescent protein; GFP; Intrabody; Membrane contact site; Mitochondria; Nanobody; RFP.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Research Progresses and Applications of Fluorescent Protein Antibodies: A Review Focusing on Nanobodies.Int J Mol Sci. 2023 Feb 21;24(5):4307. doi: 10.3390/ijms24054307. Int J Mol Sci. 2023. PMID: 36901737 Free PMC article. Review.
-
Unintended perturbation of protein function using GFP nanobodies in human cells.J Cell Sci. 2019 Nov 6;132(21):jcs234955. doi: 10.1242/jcs.234955. J Cell Sci. 2019. PMID: 31601614 Free PMC article.
-
Intracellular Delivery of Nanobodies for Imaging of Target Proteins in Live Cells.Pharm Res. 2017 Jan;34(1):161-174. doi: 10.1007/s11095-016-2052-8. Epub 2016 Oct 31. Pharm Res. 2017. PMID: 27800572
-
A versatile nanobody-based toolkit to analyze retrograde transport from the cell surface.Proc Natl Acad Sci U S A. 2018 Jul 3;115(27):E6227-E6236. doi: 10.1073/pnas.1801865115. Epub 2018 Jun 18. Proc Natl Acad Sci U S A. 2018. PMID: 29915061 Free PMC article.
-
Recombinant aequorin and green fluorescent protein as valuable tools in the study of cell signalling.Biochem J. 2001 Apr 1;355(Pt 1):1-12. doi: 10.1042/0264-6021:3550001. Biochem J. 2001. PMID: 11256942 Free PMC article. Review.
Cited by
-
Single-domain near-infrared protein provides a scaffold for antigen-dependent fluorescent nanobodies.Nat Methods. 2022 Jun;19(6):740-750. doi: 10.1038/s41592-022-01467-6. Epub 2022 May 23. Nat Methods. 2022. PMID: 35606446 Free PMC article.
-
Research Progresses and Applications of Fluorescent Protein Antibodies: A Review Focusing on Nanobodies.Int J Mol Sci. 2023 Feb 21;24(5):4307. doi: 10.3390/ijms24054307. Int J Mol Sci. 2023. PMID: 36901737 Free PMC article. Review.
-
Detection of transcriptome-wide microRNA-target interactions in single cells with agoTRIBE.Nat Biotechnol. 2024 Aug;42(8):1296-1302. doi: 10.1038/s41587-023-01951-0. Epub 2023 Sep 21. Nat Biotechnol. 2024. PMID: 37735263 Free PMC article.
-
Protein manipulation using single copies of short peptide tags in cultured cells and in Drosophila melanogaster.Development. 2021 Mar 16;148(6):dev191700. doi: 10.1242/dev.191700. Development. 2021. PMID: 33593816 Free PMC article.
-
Mandipropamid as a chemical inducer of proximity for in vivo applications.Nat Chem Biol. 2022 Jan;18(1):64-69. doi: 10.1038/s41589-021-00922-3. Epub 2021 Dec 21. Nat Chem Biol. 2022. PMID: 34934192 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

