STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3
- PMID: 31121492
- PMCID: PMC6529775
- DOI: 10.1016/j.redox.2019.101215
STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3
Abstract
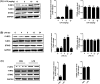
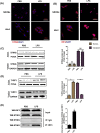
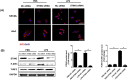
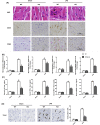
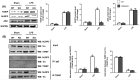
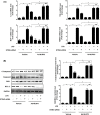
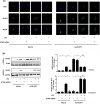
Mountainous evidence suggests that inflammation, cardiomyocyte apoptosis and pyroptosis are involved in the development of sepsis and sepsis-induced cardiomyopathy (SIC). Stimulator of interferon genes (STING) is an indispensable molecule that could regulate inflammation and immune response in multiple diseases. However, the role of STING in cardiovascular disease, especially SIC remains unclear. This study was designed to investigate the potential molecular mechanisms of STING in lipopolysaccharide (LPS)-induced cardiac injury using STING global knockout mice. In wild type mice and cardiomyocytes, LPS stimulation triggered the perinuclear translocation of STING, which further bound to Type-I interferons (IFN) regulatory factor 3 (IRF3) and phosphorylated IRF3. Phosphorylated (P-) IRF3 subsequently translocated into nucleus and increased the expression of NOD-like receptor protein 3 (NLRP3). Knockout of STING in mice significantly improved survival rate and cardiac function, apart from suppressing myocardial and serum inflammatory cytokines, apoptosis, as well as cardiomyocyte pyroptosis. In vitro experiments revealed that NLRP3 overexpression by adenovirus could offset protective effects of STING knockdown in LPS-induced cardiomyocytes. Additionally, LPS stimulation also promoted the production of intracellular reactive oxygen (ROS), which further induced the NLRP3 translocation to the cytoplasm from the nucleus. Dissociative TXNIP could directly interact with cytoplasmic NLRP3 and form inflammasome, eventually triggering cardiomyocyte injury. Collectively, our findings disclose that STING deficiency could alleviate LPS-induced SIC in mice. Hence, targeting STING in cardiomyocytes may be a promising therapeutic strategy for preventing SIC.
Keywords: Apoptosis; NLRP3 inflammasome; Pyroptosis; STING-IRF3; Sepsis-induced cardiomyopathy.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





Similar articles
-
Mitochondrial (mt)DNA-cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling promotes pyroptosis of macrophages via interferon regulatory factor (IRF)7/IRF3 activation to aggravate lung injury during severe acute pancreatitis.Cell Mol Biol Lett. 2024 Apr 27;29(1):61. doi: 10.1186/s11658-024-00575-9. Cell Mol Biol Lett. 2024. PMID: 38671352 Free PMC article.
-
Cytosolic mtDNA-cGAS-STING axis contributes to sepsis-induced acute kidney injury via activating the NLRP3 inflammasome.Clin Exp Nephrol. 2024 May;28(5):375-390. doi: 10.1007/s10157-023-02448-5. Epub 2024 Jan 19. Clin Exp Nephrol. 2024. PMID: 38238499
-
Lipopolysaccharide (LPS) Aggravates High Glucose- and Hypoxia/Reoxygenation-Induced Injury through Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis in H9C2 Cardiomyocytes.J Diabetes Res. 2019 Feb 17;2019:8151836. doi: 10.1155/2019/8151836. eCollection 2019. J Diabetes Res. 2019. PMID: 30911553 Free PMC article.
-
The cGAS-STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy.Acta Pharm Sin B. 2022 Jan;12(1):50-75. doi: 10.1016/j.apsb.2021.05.011. Epub 2021 May 20. Acta Pharm Sin B. 2022. PMID: 35127372 Free PMC article. Review.
-
When pyro(ptosis) meets palm(itoylation).Cytokine Growth Factor Rev. 2024 Jun;77:30-38. doi: 10.1016/j.cytogfr.2024.03.001. Epub 2024 Mar 4. Cytokine Growth Factor Rev. 2024. PMID: 38472042 Review.
Cited by
-
Dual-tDCS Ameliorates Cerebral Injury and Promotes Motor Function Recovery via cGAS-STING Signaling Pathway in a Rat Model of Ischemic Stroke.Mol Neurobiol. 2024 Oct 26. doi: 10.1007/s12035-024-04574-x. Online ahead of print. Mol Neurobiol. 2024. PMID: 39455539
-
Acute exposure to LPS induces cardiac dysfunction via the activation of the NLRP3 inflammasome.Sci Rep. 2024 Oct 17;14(1):24378. doi: 10.1038/s41598-024-76066-w. Sci Rep. 2024. PMID: 39420211 Free PMC article.
-
The multiple roles of interferon regulatory factor family in health and disease.Signal Transduct Target Ther. 2024 Oct 9;9(1):282. doi: 10.1038/s41392-024-01980-4. Signal Transduct Target Ther. 2024. PMID: 39384770 Free PMC article. Review.
-
Silencing of TXNIP attenuates oxidative stress injury in HEI-OC1 by inhibiting the activation of NLRP3 and NF-κB.Heliyon. 2024 Sep 10;10(18):e37753. doi: 10.1016/j.heliyon.2024.e37753. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39381226 Free PMC article.
-
Ubiquitin-independent degradation of Bim blocks macrophage pyroptosis in sepsis-related tissue injury.Cell Death Dis. 2024 Sep 30;15(9):703. doi: 10.1038/s41419-024-07072-z. Cell Death Dis. 2024. PMID: 39349939 Free PMC article.
References
-
- Liu Y.C., Yu M.M., Shou S.T., Chai Y.F. Sepsis-induced cardiomyopathy: mechanisms and treatments. Front. Immunol. 2017;8:1021. - PMC - PubMed
- Liu, Y. C., Yu, M. M., Shou, S. T. & Chai, Y. F. Sepsis-Induced Cardiomyopathy: Mechanisms and Treatments. Frontiers in Immunology 8, 1021- (2017). - PMC - PubMed
-
- Wang Y. β 1 -adrenoceptor stimulation promotes LPS-induced cardiomyocyte apoptosis through activating PKA and enhancing CaMKII and IκBα phosphorylation. Crit. Care. 2015;19:76. - PMC - PubMed
- Wang, Y. et al. β 1 -adrenoceptor stimulation promotes LPS-induced cardiomyocyte apoptosis through activating PKA and enhancing CaMKII and IκBα phosphorylation. Critical Care 19, 76 (2015). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

