Osteoblast-Derived Vesicle Protein Content Is Temporally Regulated During Osteogenesis: Implications for Regenerative Therapies
- PMID: 31119130
- PMCID: PMC6504811
- DOI: 10.3389/fbioe.2019.00092
Osteoblast-Derived Vesicle Protein Content Is Temporally Regulated During Osteogenesis: Implications for Regenerative Therapies
Erratum in
-
Corrigendum: Osteoblast-Derived Vesicle Protein Content Is Temporally Regulated During Osteogenesis: Implications for Regenerative Therapies.Front Bioeng Biotechnol. 2019 Dec 6;7:392. doi: 10.3389/fbioe.2019.00392. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31853449 Free PMC article.
Abstract
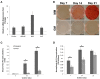
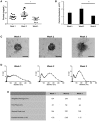
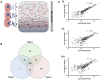
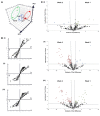
Osteoblast-derived extracellular vesicles (EV) are a collection of secreted (sEVs) and matrix-bound nanoparticles that function as foci for mineral nucleation and accumulation. Due to the fact sEVs can be isolated directly from the culture medium of mineralizing osteoblasts, there is growing interest their application regenerative medicine. However, at present therapeutic advancements are hindered by a lack of understanding of their precise temporal contribution to matrix mineralization. This study advances current knowledge by temporally aligning sEV profile and protein content with mineralization status. sEVs were isolated from mineralizing primary osteoblasts over a period of 1, 2, and 3 weeks. Bimodal particle distributions were observed (weeks 1 and 3: 44 and 164 nm; week 2: 59 and 220 nm), indicating a heterogeneous population with dimensions characteristic of exosome- (44 and 59 nm) and microvesicle-like (164 and 220 nm) particles. Proteomic characterization by liquid chromatography tandem-mass spectrometry (LC-MS/MS) revealed a declining correlation in EV-localized proteins as mineralization advanced, with Pearson correlation-coefficients of 0.79 (week 1 vs. 2), 0.6 (2 vs. 3) and 0.46 (1 vs. 3), respectively. Principal component analysis (PCA) further highlighted a time-dependent divergence in protein content as mineralization advanced. The most significant variations were observed at week 3, with a significant (p < 0.05) decline in particle concentration, visual evidence of EV rupture and enhanced mineralization. A total of 116 vesicle-localized proteins were significantly upregulated at week 3 (56% non-specifically, 19% relative to week 1, 25% relative to week 2). Gene ontology enrichment analysis of these proteins highlighted overrepresentation of genes associated with matrix organization. Of note, increased presence of phospholipid-binding and calcium channeling annexin proteins (A2, A5, and A6) indicative of progressive variations in the nucleational capacity of vesicles, as well as interaction with the surrounding ECM. We demonstrate sEV-mediated mineralization is dynamic process with variations in vesicle morphology and protein content having a potential influence on developmental changes matrix organization. These findings have implications for the selection and application of EVs for regenerative applications.
Keywords: annexin; collagen; mineralization; nano; osteoblast; vesicle.
Figures

Similar articles
-
Proteomic signatures of extracellular vesicles secreted by nonmineralizing and mineralizing human osteoblasts and stimulation of tumor cell growth.FASEB J. 2015 Jan;29(1):274-85. doi: 10.1096/fj.14-261404. Epub 2014 Oct 30. FASEB J. 2015. PMID: 25359493
-
Matrix Vesicle-Mediated Mineralization and Osteocytic Regulation of Bone Mineralization.Int J Mol Sci. 2022 Sep 1;23(17):9941. doi: 10.3390/ijms23179941. Int J Mol Sci. 2022. PMID: 36077336 Free PMC article. Review.
-
Proteomic characterization of macro-, micro- and nano-extracellular vesicles derived from the same first trimester placenta: relevance for feto-maternal communication.Hum Reprod. 2016 Apr;31(4):687-99. doi: 10.1093/humrep/dew004. Epub 2016 Feb 1. Hum Reprod. 2016. PMID: 26839151
-
Annexin-enriched osteoblast-derived vesicles act as an extracellular site of mineral nucleation within developing stem cell cultures.Sci Rep. 2017 Oct 3;7(1):12639. doi: 10.1038/s41598-017-13027-6. Sci Rep. 2017. PMID: 28974747 Free PMC article.
-
Proteomics analysis of circulating small extracellular vesicles: Focus on the contribution of EVs to tumor metabolism.Cytokine Growth Factor Rev. 2023 Oct;73:3-19. doi: 10.1016/j.cytogfr.2023.08.003. Epub 2023 Aug 19. Cytokine Growth Factor Rev. 2023. PMID: 37652834 Review.
Cited by
-
Bone Cell Exosomes and Emerging Strategies in Bone Engineering.Biomedicines. 2022 Mar 24;10(4):767. doi: 10.3390/biomedicines10040767. Biomedicines. 2022. PMID: 35453517 Free PMC article. Review.
-
AnnexinA6: a potential therapeutic target gene for extracellular matrix mineralization.Front Cell Dev Biol. 2023 Sep 4;11:1201200. doi: 10.3389/fcell.2023.1201200. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37727505 Free PMC article. Review.
-
Could BMPs Therapy Be Improved if BMPs Were Used in Composition Acting during Bone Formation in Endochondral Ossification?Int J Mol Sci. 2022 Sep 7;23(18):10327. doi: 10.3390/ijms231810327. Int J Mol Sci. 2022. PMID: 36142232 Free PMC article. Review.
-
The potential therapeutic role of extracellular vesicles in critical-size bone defects: Spring of cell-free regenerative medicine is coming.Front Bioeng Biotechnol. 2023 Jan 17;11:1050916. doi: 10.3389/fbioe.2023.1050916. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36733961 Free PMC article. Review.
-
Extracellular vesicles: From bone development to regenerative orthopedics.Mol Ther. 2023 May 3;31(5):1251-1274. doi: 10.1016/j.ymthe.2023.02.021. Epub 2023 Mar 3. Mol Ther. 2023. PMID: 36869588 Free PMC article. Review.
References
-
- Amir D., Schwartz Z., Sela J., Weinberg H. (1988). The relationship between extracellular matrix vesicles and the calcifying front on the 21st day after injury to rat tibial bone. Clin. Orthop. Relat. Res. 142–148. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

