Augmentation of HIV-specific T cell function by immediate treatment of hyperacute HIV-1 infection
- PMID: 31118290
- PMCID: PMC6901350
- DOI: 10.1126/scitranslmed.aau0528
Augmentation of HIV-specific T cell function by immediate treatment of hyperacute HIV-1 infection
Abstract
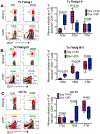
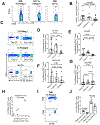
Sustained viremia after acute HIV infection is associated with profound CD4+ T cell loss and exhaustion of HIV-specific CD8+ T cell responses. To determine the impact of combination antiretroviral therapy (cART) on these processes, we examined the evolution of immune responses in acutely infected individuals initiating treatment before peak viremia. Immediate treatment of Fiebig stages I and II infection led to a rapid decline in viral load and diminished magnitude of HIV-specific (tetramer+) CD8+ T cell responses compared to untreated donors. There was a strong positive correlation between cumulative viral antigen exposure before full cART-induced suppression and immune responses measured by MHC class I tetramers, IFN-γ ELISPOT, and CD8+ T cell activation. HIV-specific CD8+ T responses of early treated individuals were characterized by increased CD127 and BCL-2 expression, greater in vitro IFN-γ secretion, and enhanced differentiation into effector memory (Tem) cells. Transcriptional analysis of tetramer+ CD8+ T cells from treated persons revealed reduced expression of genes associated with activation and apoptosis, with concurrent up-regulation of prosurvival genes including BCL-2, AXL, and SRC Early treatment also resulted in robust HIV-specific CD4+ T cell responses compared to untreated HIV-infected individuals. Our data show that limiting acute viremia results in enhanced functionality of HIV-specific CD4+ and CD8+ T cells, preserving key antiviral properties of these cells.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY).
Conflict of interest statement
Figures


Similar articles
-
Phenotype, Polyfunctionality, and Antiviral Activity of in vitro Stimulated CD8+ T-Cells From HIV+ Subjects Who Initiated cART at Different Time-Points After Acute Infection.Front Immunol. 2018 Oct 23;9:2443. doi: 10.3389/fimmu.2018.02443. eCollection 2018. Front Immunol. 2018. PMID: 30405632 Free PMC article.
-
HIV replication leads to skewed maturation of CD8-positive T-cell responses in infected children.New Microbiol. 2010 Oct;33(4):303-9. New Microbiol. 2010. PMID: 21213588
-
Partial recovery of senescence and differentiation disturbances in CD8+ T cell effector-memory cells in HIV-1 infection after initiation of anti-retroviral treatment.Clin Exp Immunol. 2016 Nov;186(2):227-238. doi: 10.1111/cei.12837. Epub 2016 Aug 23. Clin Exp Immunol. 2016. PMID: 27377704 Free PMC article.
-
HIV-specific CD8⁺ T cells and HIV eradication.J Clin Invest. 2016 Feb;126(2):455-63. doi: 10.1172/JCI80566. Epub 2016 Jan 5. J Clin Invest. 2016. PMID: 26731469 Free PMC article. Review.
-
Harnessing CD8+ T Cells Under HIV Antiretroviral Therapy.Front Immunol. 2019 Feb 26;10:291. doi: 10.3389/fimmu.2019.00291. eCollection 2019. Front Immunol. 2019. PMID: 30863403 Free PMC article. Review.
Cited by
-
The Role of Natural Killer Cells and Their Metabolism in HIV-1 Infection.Viruses. 2024 Oct 9;16(10):1584. doi: 10.3390/v16101584. Viruses. 2024. PMID: 39459918 Free PMC article. Review.
-
Immune mobilising T cell receptors redirect polyclonal CD8+ T cells in chronic HIV infection to form immunological synapses.Sci Rep. 2022 Nov 1;12(1):18366. doi: 10.1038/s41598-022-23228-3. Sci Rep. 2022. PMID: 36319836 Free PMC article.
-
What's Hot in HIV in 2019-A Basic and Translational Science Summary for Clinicians From IDWeek 2019.Open Forum Infect Dis. 2020 Feb 13;7(3):ofaa053. doi: 10.1093/ofid/ofaa053. eCollection 2020 Mar. Open Forum Infect Dis. 2020. PMID: 32154324 Free PMC article. Review.
-
Evolution and Diversity of Immune Responses during Acute HIV Infection.Immunity. 2020 Nov 17;53(5):908-924. doi: 10.1016/j.immuni.2020.10.015. Immunity. 2020. PMID: 33207216 Free PMC article. Review.
-
Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition).Eur J Immunol. 2021 Dec;51(12):2708-3145. doi: 10.1002/eji.202170126. Epub 2021 Dec 7. Eur J Immunol. 2021. PMID: 34910301 Free PMC article. Review.
References
-
- Fauci AS, Marston HD. 2015. Ending the HIV-AIDS Pandemic--Follow the Science. N Engl J Med 373:2197–2199. - PubMed
-
- Mellors JW, Rinaldo CR Jr., Gupta P, White RM, Todd JA, Kingsley LA. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167–1170. - PubMed
-
- Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, Boswell SL, Mathews WC, Bangsberg DR, Martin J, Whalen CC, Sieg S, Yadavalli S, Deeks SG, Lederman MM. 2006. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. Jama 296:1498–1506. - PubMed
-
- Lyles RH, Munoz A, Yamashita TE, Bazmi H, Detels R, Rinaldo CR, Margolick JB, Phair JP, Mellors JW. 2000. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis 181:872–880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL095791/HL/NHLBI NIH HHS/United States
- R01 HL134539/HL/NHLBI NIH HHS/United States
- R37 AI067073/AI/NIAID NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- R33 CA202820/CA/NCI NIH HHS/United States
- R01 AI145305/AI/NIAID NIH HHS/United States
- 55008743/HHMI/Howard Hughes Medical Institute/United States
- U19 AI089992/AI/NIAID NIH HHS/United States
- U24 AI118672/AI/NIAID NIH HHS/United States
- UM1 AI126617/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- RM1 HG006193/HG/NHGRI NIH HHS/United States
- U54 CA217377/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

