The extracellular gate shapes the energy profile of an ABC exporter
- PMID: 31113958
- PMCID: PMC6529423
- DOI: 10.1038/s41467-019-09892-6
The extracellular gate shapes the energy profile of an ABC exporter
Abstract
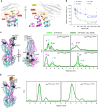
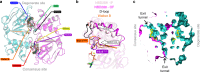
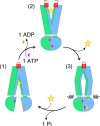
ABC exporters harness the energy of ATP to pump substrates across membranes. Extracellular gate opening and closure are key steps of the transport cycle, but the underlying mechanism is poorly understood. Here, we generated a synthetic single domain antibody (sybody) that recognizes the heterodimeric ABC exporter TM287/288 exclusively in the presence of ATP, which was essential to solve a 3.2 Å crystal structure of the outward-facing transporter. The sybody binds to an extracellular wing and strongly inhibits ATPase activity by shifting the transporter's conformational equilibrium towards the outward-facing state, as shown by double electron-electron resonance (DEER). Mutations that facilitate extracellular gate opening result in a comparable equilibrium shift and strongly reduce ATPase activity and drug transport. Using the sybody as conformational probe, we demonstrate that efficient extracellular gate closure is required to dissociate the NBD dimer after ATP hydrolysis to reset the transporter back to its inward-facing state.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Exploring conformational equilibria of a heterodimeric ABC transporter.Elife. 2017 Jan 4;6:e20236. doi: 10.7554/eLife.20236. Elife. 2017. PMID: 28051765 Free PMC article.
-
Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation.Nat Struct Mol Biol. 2012 Mar 25;19(4):395-402. doi: 10.1038/nsmb.2267. Nat Struct Mol Biol. 2012. PMID: 22447242
-
Structural Dynamics of the Heterodimeric ABC Transporter TM287/288 Induced by ATP and Substrate Binding.Biochemistry. 2016 Dec 6;55(48):6730-6738. doi: 10.1021/acs.biochem.6b00947. Epub 2016 Nov 28. Biochemistry. 2016. PMID: 27933796
-
Luminescence resonance energy transfer spectroscopy of ATP-binding cassette proteins.Biochim Biophys Acta Biomembr. 2018 Apr;1860(4):854-867. doi: 10.1016/j.bbamem.2017.08.005. Epub 2017 Aug 8. Biochim Biophys Acta Biomembr. 2018. PMID: 28801111 Review.
-
Structural basis for the mechanism of ABC transporters.Biochem Soc Trans. 2015 Oct;43(5):889-93. doi: 10.1042/BST20150047. Biochem Soc Trans. 2015. PMID: 26517899 Review.
Cited by
-
Dynamic basis of lipopolysaccharide export by LptB2FGC.Elife. 2024 Oct 7;13:RP99338. doi: 10.7554/eLife.99338. Elife. 2024. PMID: 39374147 Free PMC article.
-
The ABC transporter MsbA adopts the wide inward-open conformation in E. coli cells.Sci Adv. 2022 Oct 14;8(41):eabn6845. doi: 10.1126/sciadv.abn6845. Epub 2022 Oct 12. Sci Adv. 2022. PMID: 36223470 Free PMC article.
-
Deep mutational scan of a drug efflux pump reveals its structure-function landscape.Nat Chem Biol. 2023 Apr;19(4):440-450. doi: 10.1038/s41589-022-01205-1. Epub 2022 Nov 28. Nat Chem Biol. 2023. PMID: 36443574 Free PMC article.
-
On the interplay between lipids and asymmetric dynamics of an NBS degenerate ABC transporter.Commun Biol. 2023 Feb 3;6(1):149. doi: 10.1038/s42003-023-04537-3. Commun Biol. 2023. PMID: 36737455 Free PMC article.
-
Picky ABCG5/G8 and promiscuous ABCG2 - a tale of fatty diets and drug toxicity.FEBS Lett. 2020 Dec;594(23):4035-4058. doi: 10.1002/1873-3468.13938. Epub 2020 Oct 14. FEBS Lett. 2020. PMID: 32978801 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

