Colloidal Silver Induces Cytoskeleton Reorganization and E-Cadherin Recruitment at Cell-Cell Contacts in HaCaT Cells
- PMID: 31096606
- PMCID: PMC6631624
- DOI: 10.3390/ph12020072
Colloidal Silver Induces Cytoskeleton Reorganization and E-Cadherin Recruitment at Cell-Cell Contacts in HaCaT Cells
Abstract
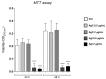
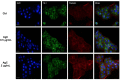
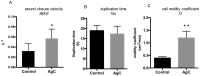
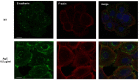
Up until the first half of the 20th century, silver found significant employment in medical applications, particularly in the healing of open wounds, thanks to its antibacterial and antifungal properties. Wound repair is a complex and dynamic biological process regulated by several pathways that cooperate to restore tissue integrity and homeostasis. To facilitate healing, injuries need to be promptly treated. Recently, the interest in alternatives to antibiotics has been raised given the widespread phenomenon of antibiotic resistance. Among these alternatives, the use of silver appears to be a valid option, so a resurgence in its use has been recently observed. In particular, in contrast to ionic silver, colloidal silver, a suspension of metallic silver particles, shows antibacterial activity displaying less or no toxicity. However, the human health risks associated with exposure to silver nanoparticles (NP) appear to be conflicted, and some studies have suggested that it could be toxic in different cellular contexts. These potentially harmful effects of silver NP depend on various parameters including NP size, which commonly range from 1 to 100 nm. In this study, we analyzed the effect of a colloidal silver preparation composed of very small and homogeneous nanoparticles of 0.62 nm size, smaller than those previously tested. We found no adverse effect on the cell proliferation of HaCaT cells, even at high NP concentration. Time-lapse microscopy and indirect immunofluorescence experiments demonstrated that this preparation of colloidal silver strongly increased cell migration, re-modeled the cytoskeleton, and caused recruitment of E-cadherin at cell-cell junctions of human cultured keratinocytes.
Keywords: E-cadherin; colloidal silver; keratinocytes; nanoparticles; skin; wound healing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Negative pressure induces p120-catenin-dependent adherens junction disassembly in keratinocytes during wound healing.Biochim Biophys Acta. 2016 Sep;1863(9):2212-20. doi: 10.1016/j.bbamcr.2016.05.017. Epub 2016 May 22. Biochim Biophys Acta. 2016. PMID: 27220534
-
Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing.ChemMedChem. 2010 Mar 1;5(3):468-75. doi: 10.1002/cmdc.200900502. ChemMedChem. 2010. PMID: 20112331
-
Metabolomics of silver nanoparticles toxicity in HaCaT cells: structure-activity relationships and role of ionic silver and oxidative stress.Nanotoxicology. 2016 Oct;10(8):1105-17. doi: 10.1080/17435390.2016.1177744. Epub 2016 May 4. Nanotoxicology. 2016. PMID: 27144425
-
PVP-coated, negatively charged silver nanoparticles: A multi-center study of their physicochemical characteristics, cell culture and in vivo experiments.Beilstein J Nanotechnol. 2014 Nov 3;5:1944-65. doi: 10.3762/bjnano.5.205. eCollection 2014. Beilstein J Nanotechnol. 2014. PMID: 25383306 Free PMC article. Review.
-
[Nanosilver--Occupational exposure limits].Med Pr. 2015;66(3):429-42. doi: 10.13075/mp.5893.00177. Med Pr. 2015. PMID: 26325054 Review. Polish.
Cited by
-
An Updated Review on Silver Nanoparticles in Biomedicine.Nanomaterials (Basel). 2020 Nov 23;10(11):2318. doi: 10.3390/nano10112318. Nanomaterials (Basel). 2020. PMID: 33238486 Free PMC article. Review.
-
Modulation of intestinal epithelial cell proliferation and apoptosis by Lactobacillus gasseri SF1183.Sci Rep. 2022 Nov 24;12(1):20248. doi: 10.1038/s41598-022-24483-0. Sci Rep. 2022. PMID: 36424419 Free PMC article.
-
Adaptive changes induced by noble-metal nanostructures in vitro and in vivo.Theranostics. 2020 Apr 27;10(13):5649-5670. doi: 10.7150/thno.42569. eCollection 2020. Theranostics. 2020. PMID: 32483410 Free PMC article. Review.
-
Mutation of the Conserved Threonine 8 within the Human ARF Tumour Suppressor Protein Regulates Autophagy.Biomolecules. 2022 Jan 13;12(1):126. doi: 10.3390/biom12010126. Biomolecules. 2022. PMID: 35053274 Free PMC article.
-
Exploring Cell Migration Mechanisms in Cancer: From Wound Healing Assays to Cellular Automata Models.Cancers (Basel). 2023 Nov 3;15(21):5284. doi: 10.3390/cancers15215284. Cancers (Basel). 2023. PMID: 37958456 Free PMC article.
References
-
- Salvioni L., Galbiati E., Collico V., Alessio G., Avvakumova S., Corsi F., Tortora P., Prosperi D., Colombo M. Negatively charged silver nanoparticles with potent antibacterial activity and reduced toxicity for pharmaceutical preparations. Int. J. Nanomed. 2017;12:2517–2530. doi: 10.2147/IJN.S127799. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

