HER2-Specific Targeted Toxin DARPin-LoPE: Immunogenicity and Antitumor Effect on Intraperitoneal Ovarian Cancer Xenograft Model
- PMID: 31096563
- PMCID: PMC6567818
- DOI: 10.3390/ijms20102399
HER2-Specific Targeted Toxin DARPin-LoPE: Immunogenicity and Antitumor Effect on Intraperitoneal Ovarian Cancer Xenograft Model
Abstract
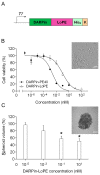
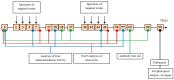
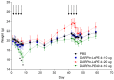
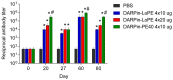
High immunogenicity and systemic toxicity are the main obstacles limiting the clinical use of the therapeutic agents based on Pseudomonas aeruginosa exotoxin A. In this work, we studied the immunogenicity, general toxicity and antitumor effect of the targeted toxin DARPin-LoPE composed of HER2-specific DARPin and a low immunogenic exotoxin A fragment lacking immunodominant human B lymphocyte epitopes. The targeted toxin has been shown to effectively inhibit the growth of HER2-positive human ovarian carcinoma xenografts, while exhibiting low non-specific toxicity and side effects, such as vascular leak syndrome and liver tissue degradation, as well as low immunogenicity, as was shown by specific antibody titer. This represents prospects for its use as an agent for targeted therapy of HER2-positive tumors.
Keywords: DARPin; HER2; Pseudomonas exotoxin A; far-red fluorescent protein; immunogenicity; ovarian carcinoma; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
[Bifunctional Toxin DARP-LoPE Based on the HER2-Specific Innovative Module of a Non-Immunoglobulin Scaffold as a Promising Agent for Theranostics].Mol Biol (Mosk). 2017 Nov-Dec;51(6):997-1007. doi: 10.7868/S0026898417060118. Mol Biol (Mosk). 2017. PMID: 29271963 Russian.
-
HER2-targeted immunotoxins with low nonspecific toxicity and immunogenicity.Biochem Biophys Res Commun. 2016 Jun 17;475(1):93-9. doi: 10.1016/j.bbrc.2016.05.044. Epub 2016 May 10. Biochem Biophys Res Commun. 2016. PMID: 27178207
-
Recombinant targeted toxin based on HER2-specific DARPin possesses a strong selective cytotoxic effect in vitro and a potent antitumor activity in vivo.J Control Release. 2016 Jul 10;233:48-56. doi: 10.1016/j.jconrel.2016.05.020. Epub 2016 May 10. J Control Release. 2016. PMID: 27178808
-
A guide to taming a toxin--recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer.FEBS J. 2011 Dec;278(23):4683-700. doi: 10.1111/j.1742-4658.2011.08182.x. Epub 2011 Jun 2. FEBS J. 2011. PMID: 21585657 Free PMC article. Review.
-
Mechanisms of Resistance to Immunotoxins Containing Pseudomonas Exotoxin A in Cancer Therapy.Biomolecules. 2020 Jun 30;10(7):979. doi: 10.3390/biom10070979. Biomolecules. 2020. PMID: 32630017 Free PMC article. Review.
Cited by
-
Natural and Designed Toxins for Precise Therapy: Modern Approaches in Experimental Oncology.Int J Mol Sci. 2021 May 7;22(9):4975. doi: 10.3390/ijms22094975. Int J Mol Sci. 2021. PMID: 34067057 Free PMC article. Review.
-
Pseudomonas aeruginosa in Cancer Therapy: Current Knowledge, Challenges and Future Perspectives.Front Oncol. 2022 Apr 28;12:891187. doi: 10.3389/fonc.2022.891187. eCollection 2022. Front Oncol. 2022. PMID: 35574361 Free PMC article. Review.
-
Epithelial cell adhesion molecule‑targeting designed ankyrin repeat protein‑toxin fusion Ec1‑LoPE exhibits potent cytotoxic action in prostate cancer cells.Oncol Rep. 2022 May;47(5):94. doi: 10.3892/or.2022.8305. Epub 2022 Mar 22. Oncol Rep. 2022. PMID: 35315504 Free PMC article.
-
3D Models of Cellular Spheroids As a Universal Tool for Studying the Cytotoxic Properties of Anticancer Compounds In Vitro.Acta Naturae. 2022 Jan-Mar;14(1):92-100. doi: 10.32607/actanaturae.11603. Acta Naturae. 2022. PMID: 35441052 Free PMC article.
-
Imaging-Guided Therapy Simultaneously Targeting HER2 and EpCAM with Trastuzumab and EpCAM-Directed Toxin Provides Additive Effect in Ovarian Cancer Model.Cancers (Basel). 2021 Aug 4;13(16):3939. doi: 10.3390/cancers13163939. Cancers (Basel). 2021. PMID: 34439094 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

