Capsaicin induces cytotoxicity in human osteosarcoma MG63 cells through TRPV1-dependent and -independent pathways
- PMID: 31095448
- PMCID: PMC6592244
- DOI: 10.1080/15384101.2019.1618119
Capsaicin induces cytotoxicity in human osteosarcoma MG63 cells through TRPV1-dependent and -independent pathways
Abstract
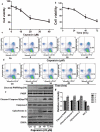
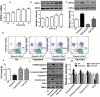
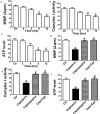
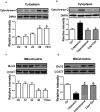
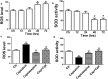
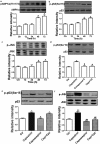
An accumulating body of evidence has shown that capsaicin induces apoptosis in various tumor cells as a mechanism of its anti-tumor activity. However, the effects of capsaicin on osteosarcoma have not been studied extensively. In the current study, we explore the molecular mechanism of capsaicin-mediated tumor suppressive function in osteosarcoma. We found that capsaicin-induced apoptosis and the activation of transient receptor potential receptor vanilloid 1 (TRPV1) in a dose- and time-dependent manner in human osteosarcoma MG63 cells in vitro. Blocking TRPV1 using capsazepine attenuated the capsaicin-induced cytotoxicity, mitochondrial dysfunction, overproduction of reactive oxygen species (ROS) and decrease in superoxide dismutase (SOD) activity. In addition, the results demonstrated that capsaicin induced the activation of adenosine 5'-monophosphate-activated protein kinase (AMPK), p53 and C-jun N-terminal kinase (JNK). In addition, Compound C (antagonist of AMPK) attenuated the activation of p53, which appeared to be TRPV1 independent. Taken together, the present study suggests that capsaicin effectively causes cell death in human osteosarcoma MG63 cells via the activation of TRPV1-dependent (mitochondrial dysfunction, and overproduction of ROS and JNK) and TRPV1-independent (AMPK-p53) pathways. Thus, capsaicin may be a potential anti-osteosarcoma agent.
Keywords: Osteosarcoma; TRPV1; apoptosis; capsaicin; mitochondria.
Figures
Similar articles
-
Capsaicin induces apoptosis in human osteosarcoma cells through AMPK-dependent and AMPK-independent signaling pathways.Mol Cell Biochem. 2013 Dec;384(1-2):229-37. doi: 10.1007/s11010-013-1802-8. Epub 2013 Sep 5. Mol Cell Biochem. 2013. PMID: 24005536
-
Capsaicin induces cytotoxicity in pancreatic neuroendocrine tumor cells via mitochondrial action.Cell Signal. 2014 Jan;26(1):41-8. doi: 10.1016/j.cellsig.2013.09.014. Epub 2013 Sep 27. Cell Signal. 2014. PMID: 24075930
-
Hypericum perforatum Attenuates Spinal Cord Injury-Induced Oxidative Stress and Apoptosis in the Dorsal Root Ganglion of Rats: Involvement of TRPM2 and TRPV1 Channels.Mol Neurobiol. 2016 Aug;53(6):3540-3551. doi: 10.1007/s12035-015-9292-1. Epub 2015 Jun 23. Mol Neurobiol. 2016. PMID: 26099309
-
Pleiotropic Pharmacological Actions of Capsazepine, a Synthetic Analogue of Capsaicin, against Various Cancers and Inflammatory Diseases.Molecules. 2019 Mar 12;24(5):995. doi: 10.3390/molecules24050995. Molecules. 2019. PMID: 30871017 Free PMC article. Review.
-
Changes in TRPV1-Mediated Physiological Function in Rats Systemically Treated With Capsaicin on the Neonate.Int J Mol Sci. 2020 Apr 29;21(9):3143. doi: 10.3390/ijms21093143. Int J Mol Sci. 2020. PMID: 32365623 Free PMC article. Review.
Cited by
-
Big Data-Based Identification of Multi-Gene Prognostic Signatures in Liver Cancer.Front Oncol. 2020 May 28;10:847. doi: 10.3389/fonc.2020.00847. eCollection 2020. Front Oncol. 2020. PMID: 32547951 Free PMC article.
-
Phytochemical Modulation of Ion Channels in Oncologic Symptomatology and Treatment.Cancers (Basel). 2024 May 6;16(9):1786. doi: 10.3390/cancers16091786. Cancers (Basel). 2024. PMID: 38730738 Free PMC article. Review.
-
Mechanism underlying the negative inotropic effect in rat left ventricle in hyperthermia: the role of TRPV1.J Physiol Sci. 2020 Feb 5;70(1):4. doi: 10.1186/s12576-020-00734-5. J Physiol Sci. 2020. PMID: 32039693 Free PMC article.
-
The p53-mediated cell cycle regulation is a potential mechanism for emodin-suppressing osteosarcoma cells.Heliyon. 2024 Feb 23;10(5):e26850. doi: 10.1016/j.heliyon.2024.e26850. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38495151 Free PMC article.
-
Capsaicin exerts synergistic pro-apoptotic effects with cisplatin in TSCC through the calpain pathway via TRPV1.J Cancer. 2024 Jul 9;15(15):4801-4817. doi: 10.7150/jca.98075. eCollection 2024. J Cancer. 2024. PMID: 39132151 Free PMC article.
References
-
- Harrison DJ, Geller DS, Gill JD, et al. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18:39–50. - PubMed
-
- Hayden JB, Hoang BH.. Osteosarcoma: basic science and clinical implications. Orthop Clin North Am. 2006;37:1–7. - PubMed
-
- Biazzo A, De Paolis M. Multidisciplinary approach to osteosarcoma. Acta Orthop Belg. 2016;82:690–698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
