Rab46 integrates Ca2+ and histamine signaling to regulate selective cargo release from Weibel-Palade bodies
- PMID: 31092558
- PMCID: PMC6605797
- DOI: 10.1083/jcb.201810118
Rab46 integrates Ca2+ and histamine signaling to regulate selective cargo release from Weibel-Palade bodies
Abstract
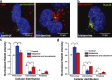
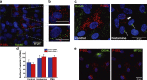
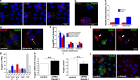
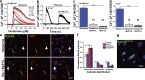
Endothelial cells selectively release cargo stored in Weibel-Palade bodies (WPBs) to regulate vascular function, but the underlying mechanisms are poorly understood. Here we show that histamine evokes the release of the proinflammatory ligand, P-selectin, while diverting WPBs carrying non-inflammatory cargo away from the plasma membrane to the microtubule organizing center. This differential trafficking is dependent on Rab46 (CRACR2A), a newly identified Ca2+-sensing GTPase, which localizes to a subset of P-selectin-negative WPBs. After acute stimulation of the H1 receptor, GTP-bound Rab46 evokes dynein-dependent retrograde transport of a subset of WPBs along microtubules. Upon continued histamine stimulation, Rab46 senses localized elevations of intracellular calcium and evokes dispersal of microtubule organizing center-clustered WPBs. These data demonstrate for the first time that a Rab GTPase, Rab46, integrates G protein and Ca2+ signals to couple on-demand histamine signals to selective WPB trafficking.
© 2019 Miteva et al.
Figures


Similar articles
-
Actin dynamics during Ca2+-dependent exocytosis of endothelial Weibel-Palade bodies.Biochim Biophys Acta Mol Cell Res. 2019 Jul;1866(7):1218-1229. doi: 10.1016/j.bbamcr.2018.11.010. Epub 2018 Nov 20. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30465794
-
Rab35 protein regulates evoked exocytosis of endothelial Weibel-Palade bodies.J Biol Chem. 2017 Jul 14;292(28):11631-11640. doi: 10.1074/jbc.M116.773333. Epub 2017 May 31. J Biol Chem. 2017. PMID: 28566286 Free PMC article.
-
Synaptotagmin 5 regulates Ca2+-dependent Weibel-Palade body exocytosis in human endothelial cells.J Cell Sci. 2019 Feb 15;132(5):jcs221952. doi: 10.1242/jcs.221952. J Cell Sci. 2019. PMID: 30659119 Free PMC article.
-
Dynamics and plasticity of Weibel-Palade bodies in endothelial cells.Arterioscler Thromb Vasc Biol. 2006 May;26(5):1002-7. doi: 10.1161/01.ATV.0000209501.56852.6c. Epub 2006 Feb 9. Arterioscler Thromb Vasc Biol. 2006. PMID: 16469951 Review.
-
How to roll an endothelial cigar: the biogenesis of Weibel-Palade bodies.Traffic. 2004 Feb;5(2):69-78. doi: 10.1111/j.1600-0854.2004.00157.x. Traffic. 2004. PMID: 14690496 Review.
Cited by
-
The Role of Rab GTPases in the development of genetic and malignant diseases.Mol Cell Biochem. 2024 Feb;479(2):255-281. doi: 10.1007/s11010-023-04727-x. Epub 2023 Apr 15. Mol Cell Biochem. 2024. PMID: 37060515 Review.
-
Super-Resolution Analysis of the Origins of the Elementary Events of ER Calcium Release in Dorsal Root Ganglion Neurons.Cells. 2023 Dec 23;13(1):38. doi: 10.3390/cells13010038. Cells. 2023. PMID: 38201242 Free PMC article.
-
Plasma membrane phosphatidylinositol (4,5)-bisphosphate promotes Weibel-Palade body exocytosis.Life Sci Alliance. 2020 Aug 21;3(11):e202000788. doi: 10.26508/lsa.202000788. Print 2020 Nov. Life Sci Alliance. 2020. PMID: 32826291 Free PMC article.
-
Weibel Palade Bodies: Unique Secretory Organelles of Endothelial Cells that Control Blood Vessel Homeostasis.Front Cell Dev Biol. 2021 Dec 16;9:813995. doi: 10.3389/fcell.2021.813995. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34977047 Free PMC article. Review.
-
Large Rab GTPases: Novel Membrane Trafficking Regulators with a Calcium Sensor and Functional Domains.Int J Mol Sci. 2021 Jul 19;22(14):7691. doi: 10.3390/ijms22147691. Int J Mol Sci. 2021. PMID: 34299309 Free PMC article. Review.
References
-
- Bonfanti R., Furie B.C., Furie B., and Wagner D.D.. 1989. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 73:1109–1112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

