The ginsenoside Rk3 exerts anti-esophageal cancer activity in vitro and in vivo by mediating apoptosis and autophagy through regulation of the PI3K/Akt/mTOR pathway
- PMID: 31091245
- PMCID: PMC6519821
- DOI: 10.1371/journal.pone.0216759
The ginsenoside Rk3 exerts anti-esophageal cancer activity in vitro and in vivo by mediating apoptosis and autophagy through regulation of the PI3K/Akt/mTOR pathway
Abstract
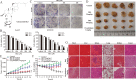
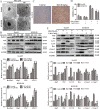
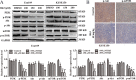
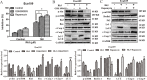
The rare ginsenoside Rk3 is a bioactive component derived from ginseng and Panax notoginseng that has been proven to possess anti-lung cancer activity. However, the effect of Rk3 on human esophageal cancer has not yet been reported. In this study, we aimed to explore its anticancer curative effect and potential molecular mechanisms in the Eca109 and KYSE150 cell lines. We found that Rk3 was able to significantly repress cell proliferation and colony formation in both Eca109 and KYSE150 cells in vitro. In the KYSE150 xenograft model, Rk3 obviously inhibited tumor growth and exhibited little toxicity in organs. Moreover, Rk3 could trigger G1 phase arrest and induce apoptosis and autophagy. Interestingly, apoptosis induced by Rk3 could be partly abrogated by 3-MA (an autophagy inhibitor), implying that autophagy could enhance apoptosis. Further studies indicated that pretreatment with the Akt inhibitor GSK690693 or the mTOR inhibitor rapamycin promoted Rk3-induced apoptosis and autophagy, demonstrating that the PI3K/Akt/mTOR pathway is related to Rk3-induced apoptosis and autophagy. In conclusion, the present study is the first to clarify that Rk3 can inhibit Eca109 and KYSE150 cell proliferation through activating apoptosis and autophagy by blocking the PI3K/Akt/mTOR pathway, suggesting that Rk3 may be a promising antitumor agent for esophageal cancer. In addition, this study provides ideas and an experimental basis for further research on the anti-esophageal cancer effects of the ginsenoside Rk3 and its mechanism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Oridonin-induced mitochondria-dependent apoptosis in esophageal cancer cells by inhibiting PI3K/AKT/mTOR and Ras/Raf pathways.J Cell Biochem. 2019 Mar;120(3):3736-3746. doi: 10.1002/jcb.27654. Epub 2018 Sep 19. J Cell Biochem. 2019. PMID: 30229997
-
Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway.Biomed Pharmacother. 2020 Feb;122:109726. doi: 10.1016/j.biopha.2019.109726. Epub 2019 Dec 30. Biomed Pharmacother. 2020. PMID: 31918283
-
Ginsenoside Rk1 induces cell cycle arrest and apoptosis in MDA-MB-231 triple negative breast cancer cells.Toxicology. 2019 Apr 15;418:22-31. doi: 10.1016/j.tox.2019.02.010. Epub 2019 Feb 21. Toxicology. 2019. PMID: 30797898
-
Anticancer property of ginsenoside Rh2 from ginseng.Eur J Med Chem. 2020 Oct 1;203:112627. doi: 10.1016/j.ejmech.2020.112627. Epub 2020 Jul 13. Eur J Med Chem. 2020. PMID: 32702586 Review.
-
Autophagy: novel applications of nonsteroidal anti-inflammatory drugs for primary cancer.Cancer Med. 2018 Feb;7(2):471-484. doi: 10.1002/cam4.1287. Epub 2017 Dec 28. Cancer Med. 2018. PMID: 29282893 Free PMC article. Review.
Cited by
-
Development and validation of an autophagy-related prognostic signature in esophageal cancer.Ann Transl Med. 2021 Feb;9(4):317. doi: 10.21037/atm-20-4541. Ann Transl Med. 2021. PMID: 33708944 Free PMC article.
-
Paving the Road Toward Exploiting the Therapeutic Effects of Ginsenosides: An Emphasis on Autophagy and Endoplasmic Reticulum Stress.Adv Exp Med Biol. 2021;1308:137-160. doi: 10.1007/978-3-030-64872-5_12. Adv Exp Med Biol. 2021. PMID: 33861443
-
Anticancer activities of phytoconstituents and their liposomal targeting strategies against tumor cells and the microenvironment.Adv Drug Deliv Rev. 2020;154-155:245-273. doi: 10.1016/j.addr.2020.05.006. Epub 2020 May 28. Adv Drug Deliv Rev. 2020. PMID: 32473991 Free PMC article. Review.
-
A recombinant scFv antibody-based fusion protein that targets EGFR associated with IMPDH2 downregulation and its drug conjugate show therapeutic efficacy against esophageal cancer.Drug Deliv. 2022 Dec;29(1):1243-1256. doi: 10.1080/10717544.2022.2063454. Drug Deliv. 2022. PMID: 35416106 Free PMC article.
-
Targeting Autophagy for Overcoming Resistance to Anti-EGFR Treatments.Cancers (Basel). 2019 Sep 16;11(9):1374. doi: 10.3390/cancers11091374. Cancers (Basel). 2019. PMID: 31527477 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

