Spindle-F-actin interactions in mitotic spindles in an intact vertebrate epithelium
- PMID: 31091161
- PMCID: PMC6727749
- DOI: 10.1091/mbc.E19-02-0126
Spindle-F-actin interactions in mitotic spindles in an intact vertebrate epithelium
Abstract
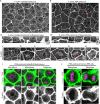
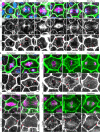
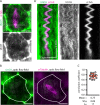
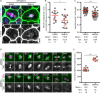
Mitotic spindles are well known to be assembled from and dependent on microtubules. In contrast, whether actin filaments (F-actin) are required for or are even present in mitotic spindles has long been controversial. Here we have developed improved methods for simultaneously preserving F-actin and microtubules in fixed samples and exploited them to demonstrate that F-actin is indeed associated with mitotic spindles in intact Xenopus laevis embryonic epithelia. We also find that there is an "F-actin cycle," in which the distribution and organization of spindle F-actin changes over the course of the cell cycle. Live imaging using a probe for F-actin reveals that at least two pools of F-actin are associated with mitotic spindles: a relatively stable internal network of cables that moves in concert with and appears to be linked to spindles, and F-actin "fingers" that rapidly extend from the cell cortex toward the spindle and make transient contact with the spindle poles. We conclude that there is a robust endoplasmic F-actin network in normal vertebrate epithelial cells and that this network is also a component of mitotic spindles. More broadly, we conclude that there is far more internal F-actin in epithelial cells than is commonly believed.
Figures

Similar articles
-
Automated mitotic spindle tracking suggests a link between spindle dynamics, spindle orientation, and anaphase onset in epithelial cells.Mol Biol Cell. 2017 Mar 15;28(6):746-759. doi: 10.1091/mbc.E16-06-0355. Epub 2017 Jan 18. Mol Biol Cell. 2017. PMID: 28100633 Free PMC article.
-
The role of actin and myosin in PtK2 spindle length changes induced by laser microbeam irradiations across the spindle.Cytoskeleton (Hoboken). 2013 May;70(5):241-59. doi: 10.1002/cm.21104. Epub 2013 Apr 10. Cytoskeleton (Hoboken). 2013. PMID: 23475753
-
F-actin is required for spindle anchoring and rotation in Xenopus oocytes: a re-examination of the effects of cytochalasin B on oocyte maturation.Zygote. 1995 Feb;3(1):17-26. doi: 10.1017/s0967199400002331. Zygote. 1995. PMID: 7613871
-
Actin-based spindle positioning: new insights from female gametes.J Cell Sci. 2014 Feb 1;127(Pt 3):477-83. doi: 10.1242/jcs.142711. Epub 2014 Jan 10. J Cell Sci. 2014. PMID: 24413163 Review.
-
And the dead shall rise: actin and myosin return to the spindle.Dev Cell. 2011 Sep 13;21(3):410-9. doi: 10.1016/j.devcel.2011.07.018. Dev Cell. 2011. PMID: 21920311 Free PMC article. Review.
Cited by
-
The myosin regulatory light chain Myl5 localizes to mitotic spindle poles and is required for proper cell division.Cytoskeleton (Hoboken). 2021 Feb;78(2):23-35. doi: 10.1002/cm.21654. Epub 2021 Mar 8. Cytoskeleton (Hoboken). 2021. PMID: 33641240 Free PMC article.
-
Mechanisms underlying Myosin 10's contribution to the maintenance of mitotic spindle bipolarity.Mol Biol Cell. 2024 Feb 1;35(2):ar14. doi: 10.1091/mbc.E23-07-0282. Epub 2023 Nov 29. Mol Biol Cell. 2024. PMID: 38019611 Free PMC article.
-
Regulation of organelle size and organization during development.Semin Cell Dev Biol. 2023 Jan 15;133:53-64. doi: 10.1016/j.semcdb.2022.02.002. Epub 2022 Feb 8. Semin Cell Dev Biol. 2023. PMID: 35148938 Free PMC article. Review.
-
Artificially decreasing cortical tension generates aneuploidy in mouse oocytes.Nat Commun. 2020 Apr 3;11(1):1649. doi: 10.1038/s41467-020-15470-y. Nat Commun. 2020. PMID: 32245998 Free PMC article.
-
Disc and Actin Associated Protein 1 influences attachment in the intestinal parasite Giardia lamblia.PLoS Pathog. 2022 Mar 25;18(3):e1010433. doi: 10.1371/journal.ppat.1010433. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35333908 Free PMC article.
References
-
- Abràmoff MD, Niessen WJ, Viergever MA. (2000). Objective quantification of the motion of soft tissues: an application to orbital soft tissue motion. IEEE Trans Med Imag , 986–995. - PubMed
-
- Aubin JE, Weber K, Osborn M. (1979). Analysis of actin and microfilament-associated proteins in the mitotic spindle and cleavage furrow of PtK2 cells by immunofluorescence microscopy. A critical note. Exp Cell Res , 93–109. - PubMed
-
- Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac MH. (2008). Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol , 1514–1519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

