Pharmacologic Approaches for Adapting Proteostasis in the Secretory Pathway to Ameliorate Protein Conformational Diseases
- PMID: 31088828
- PMCID: PMC7197434
- DOI: 10.1101/cshperspect.a034108
Pharmacologic Approaches for Adapting Proteostasis in the Secretory Pathway to Ameliorate Protein Conformational Diseases
Abstract
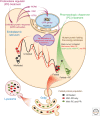
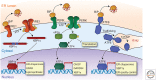
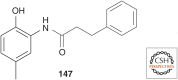
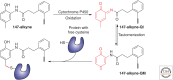
Maintenance of the proteome, ensuring the proper locations, proper conformations, appropriate concentrations, etc., is essential to preserve the health of an organism in the face of environmental insults, infectious diseases, and the challenges associated with aging. Maintaining the proteome is even more difficult in the background of inherited mutations that render a given protein and others handled by the same proteostasis machinery misfolding prone and/or aggregation prone. Maintenance of the proteome or maintaining proteostasis requires the orchestration of protein synthesis, folding, trafficking, and degradation by way of highly conserved, interacting, and competitive proteostasis pathways. Each subcellular compartment has a unique proteostasis network compromising common and specialized proteostasis maintenance pathways. Stress-responsive signaling pathways detect the misfolding and/or aggregation of proteins in specific subcellular compartments using stress sensors and respond by generating an active transcription factor. Subsequent transcriptional programs up-regulate proteostasis network capacity (i.e., ability to fold and degrade proteins in that compartment). Stress-responsive signaling pathways can also be linked by way of signaling cascades to nontranscriptional means to reestablish proteostasis (e.g., by translational attenuation). Proteostasis is also strongly influenced by the inherent kinetics and thermodynamics of the folding, misfolding, and aggregation of individual proteins, and these sequence-based attributes in combination with proteostasis network capacity together influence proteostasis. In this review, we will focus on the growing body of evidence that proteostasis deficits leading to human pathology can be reversed by pharmacologic adaptation of proteostasis network capacity through stress-responsive signaling pathway activation. The power of this approach will be exemplified by focusing on the ATF6 arm of the unfolded protein response stress responsive-signaling pathway that regulates proteostasis network capacity of the secretory pathway.
Copyright © 2020 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Similar articles
-
Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis.Cold Spring Harb Perspect Biol. 2011 Dec 1;3(12):a004507. doi: 10.1101/cshperspect.a004507. Cold Spring Harb Perspect Biol. 2011. PMID: 21900404 Free PMC article. Review.
-
Underlying mechanisms and chemical/biochemical therapeutic approaches to ameliorate protein misfolding neurodegenerative diseases.Biofactors. 2017 Nov;43(6):737-759. doi: 10.1002/biof.1264. Epub 2016 Feb 22. Biofactors. 2017. PMID: 26899445 Review.
-
Regulating Secretory Proteostasis through the Unfolded Protein Response: From Function to Therapy.Trends Cell Biol. 2017 Oct;27(10):722-737. doi: 10.1016/j.tcb.2017.05.006. Epub 2017 Jun 21. Trends Cell Biol. 2017. PMID: 28647092 Free PMC article. Review.
-
Stress-responsive regulation of extracellular proteostasis.J Cell Biol. 2022 Apr 4;221(4):e202112104. doi: 10.1083/jcb.202112104. Epub 2022 Feb 22. J Cell Biol. 2022. PMID: 35191945 Free PMC article. Review.
-
The disturbance of protein synthesis/degradation homeostasis is a common trait of age-related neurodegenerative disorders.Adv Protein Chem Struct Biol. 2022;132:49-87. doi: 10.1016/bs.apcsb.2022.05.008. Epub 2022 Jun 9. Adv Protein Chem Struct Biol. 2022. PMID: 36088079
Cited by
-
Pharmacological chaperones restore proteostasis of epilepsy-associated GABAA receptor variants.Pharmacol Res. 2024 Oct;208:107356. doi: 10.1016/j.phrs.2024.107356. Epub 2024 Aug 30. Pharmacol Res. 2024. PMID: 39216838 Free PMC article.
-
Neuronal CBP-1 is Required for Enhanced Body Muscle Proteostasis in Response to Reduced Translation Downstream of mTOR.Front Biosci (Landmark Ed). 2024 Jul 23;29(7):264. doi: 10.31083/j.fbl2907264. Front Biosci (Landmark Ed). 2024. PMID: 39082355 Free PMC article.
-
Hsp47 promotes biogenesis of multi-subunit neuroreceptors in the endoplasmic reticulum.Elife. 2024 Jul 4;13:e84798. doi: 10.7554/eLife.84798. Elife. 2024. PMID: 38963323 Free PMC article.
-
Tracing genetic diversity captures the molecular basis of misfolding disease.Nat Commun. 2024 Apr 18;15(1):3333. doi: 10.1038/s41467-024-47520-0. Nat Commun. 2024. PMID: 38637533 Free PMC article.
-
The generation of detergent-insoluble clipped fragments from an ERAD substrate in mammalian cells.Sci Rep. 2023 Dec 6;13(1):21508. doi: 10.1038/s41598-023-48769-z. Sci Rep. 2023. PMID: 38057493 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
