Osteocyte TSC1 promotes sclerostin secretion to restrain osteogenesis in mice
- PMID: 31088250
- PMCID: PMC6544986
- DOI: 10.1098/rsob.180262
Osteocyte TSC1 promotes sclerostin secretion to restrain osteogenesis in mice
Abstract
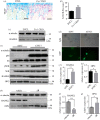
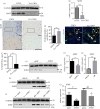
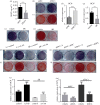
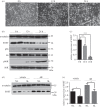
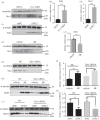
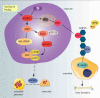
Osteocytes secrete the glycoprotein sclerostin to inhibit bone formation by osteoblasts, but how sclerostin production is regulated in osteocytes remains unclear. Here, we show that tuberous sclerosis complex 1 (TSC1) in osteocytes promotes sclerostin secretion through inhibition of mechanistic target of rapamycin complex 1 (mTORC1) and downregulation of Sirt1. We generated mice with DMP1-Cre-directed Tsc1 gene deletion ( Tsc1 CKO) to constitutively activate mTORC1 in osteocytes. Although osteocyte TSC1 disruption increased RANKL expression and osteoclast formation, it markedly reduced sclerostin production in bone, resulting in severe osteosclerosis with enhanced bone formation in mice. Knockdown of TSC1 activated mTORC1 and decreased sclerostin, while rapamycin inhibited mTORC1 and increased sclerostin mRNA and protein expression levels in MLO-Y4 osteocyte-like cells. Furthermore, mechanical loading activated mTORC1 and prevented sclerostin expression in osteocytes. Mechanistically, TSC1 promotes sclerostin production and prevents osteogenesis through inhibition of mTORC1 and downregulation of Sirt1, a repressor of the sclerostin gene Sost. Our findings reveal a role of TSC1/mTORC1 signalling in the regulation of osteocyte sclerostin secretion and bone formation in response to mechanical loading in vitro. Targeting TSC1 represents a potential strategy to increase osteogenesis and prevent bone loss-related diseases.
Keywords: Sirt1; TSC1; mTORC1; osteocyte; osteogenesis; sclerostin.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures


Similar articles
-
TSC1 regulates osteoclast podosome organization and bone resorption through mTORC1 and Rac1/Cdc42.Cell Death Differ. 2018 Sep;25(9):1549-1566. doi: 10.1038/s41418-017-0049-4. Epub 2018 Jan 22. Cell Death Differ. 2018. PMID: 29358671 Free PMC article.
-
Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway.PLoS One. 2011;6(10):e25900. doi: 10.1371/journal.pone.0025900. Epub 2011 Oct 4. PLoS One. 2011. PMID: 21991382 Free PMC article.
-
Iron overload induced osteocytes apoptosis and led to bone loss in Hepcidin-/- mice through increasing sclerostin and RANKL/OPG.Bone. 2022 Nov;164:116511. doi: 10.1016/j.bone.2022.116511. Epub 2022 Aug 4. Bone. 2022. PMID: 35933095
-
Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption.Int J Mol Sci. 2020 Jul 21;21(14):5169. doi: 10.3390/ijms21145169. Int J Mol Sci. 2020. PMID: 32708317 Free PMC article. Review.
-
Osteocyte control of bone remodeling: is sclerostin a key molecular coordinator of the balanced bone resorption-formation cycles?Osteoporos Int. 2014 Dec;25(12):2685-700. doi: 10.1007/s00198-014-2808-0. Epub 2014 Jul 17. Osteoporos Int. 2014. PMID: 25030653 Review.
Cited by
-
Endocrine role of bone in the regulation of energy metabolism.Bone Res. 2021 May 20;9(1):25. doi: 10.1038/s41413-021-00142-4. Bone Res. 2021. PMID: 34016950 Free PMC article. Review.
-
Role of TSC1 in physiology and diseases.Mol Cell Biochem. 2021 Jun;476(6):2269-2282. doi: 10.1007/s11010-021-04088-3. Epub 2021 Feb 11. Mol Cell Biochem. 2021. PMID: 33575875 Review.
-
Heterotopic Ossification: Clinical Features, Basic Researches, and Mechanical Stimulations.Front Cell Dev Biol. 2022 Jan 25;10:770931. doi: 10.3389/fcell.2022.770931. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35145964 Free PMC article. Review.
-
New Advances in Osteocyte Mechanotransduction.Curr Osteoporos Rep. 2021 Feb;19(1):101-106. doi: 10.1007/s11914-020-00650-y. Epub 2021 Jan 9. Curr Osteoporos Rep. 2021. PMID: 33420631 Free PMC article. Review.
-
Neuronal Induction of Bone-Fat Imbalance through Osteocyte Neuropeptide Y.Adv Sci (Weinh). 2021 Dec;8(24):e2100808. doi: 10.1002/advs.202100808. Epub 2021 Oct 31. Adv Sci (Weinh). 2021. PMID: 34719888 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

