Histone Deacetylase Inhibitors Enhance Cell Killing and Block Interferon-Beta Synthesis Elicited by Infection with an Oncolytic Parainfluenza Virus
- PMID: 31083335
- PMCID: PMC6563284
- DOI: 10.3390/v11050431
Histone Deacetylase Inhibitors Enhance Cell Killing and Block Interferon-Beta Synthesis Elicited by Infection with an Oncolytic Parainfluenza Virus
Abstract
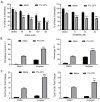
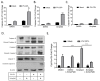
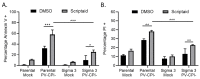
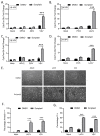
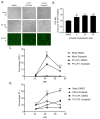
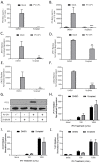
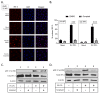
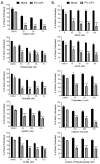
Previous results have shown that infection with the cytoplasmic-replicating parainfluenza virus 5 mutant P/V-CPI- sensitizes cells to DNA damaging agents, resulting in the enhanced killing of airway cancer cells. Here, we have tested the hypothesis that histone deacetylase (HDAC) inhibitors can also act with P/V-CPI- infection to enhance cancer cell killing. Using human small cell lung cancer and laryngeal cancer cell lines, 10 HDAC inhibitors were tested for their effect on viability of P/V-CPI- infected cells. HDAC inhibitors such as scriptaid enhanced caspase-3/7, -8 and -9 activity induced by P/V-CPI- and overall cell toxicity. Scriptaid-mediated enhanced killing was eliminated in lung cancer cells that were engineered to express a protein which sequesters double stranded RNA. Scriptaid also enhanced cancer cell killing by two other negative strand RNA viruses - the La Crosse virus and vesicular stomatitis virus. Scriptaid treatment enhanced the spread of the P/V-CPI- virus through a population of cancer cells, and suppressed interferon-beta induction through blocking phosphorylation and nuclear translocation of Interferon Regulatory Factor 3 (IRF-3). Taken together, these data support a role for combinations of a cytoplasmic-replicating RNA virus such as the P/V-CPI- mutant along with chemotherapeutic agents.
Keywords: histone deacetylase inhibitors; oncolytic virus; parainfluenza virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A hyperfusogenic F protein enhances the oncolytic potency of a paramyxovirus simian virus 5 P/V mutant without compromising sensitivity to type I interferon.J Virol. 2008 Oct;82(19):9369-80. doi: 10.1128/JVI.01054-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667520 Free PMC article.
-
Parainfluenza Virus Infection Sensitizes Cancer Cells to DNA-Damaging Agents: Implications for Oncolytic Virus Therapy.J Virol. 2018 Mar 14;92(7):e01948-17. doi: 10.1128/JVI.01948-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29343567 Free PMC article.
-
The HDAC Inhibitors Scriptaid and LBH589 Combined with the Oncolytic Virus Delta24-RGD Exert Enhanced Anti-Tumor Efficacy in Patient-Derived Glioblastoma Cells.PLoS One. 2015 May 18;10(5):e0127058. doi: 10.1371/journal.pone.0127058. eCollection 2015. PLoS One. 2015. PMID: 25993039 Free PMC article.
-
Overcoming Barriers in Oncolytic Virotherapy with HDAC Inhibitors and Immune Checkpoint Blockade.Viruses. 2016 Jan 6;8(1):9. doi: 10.3390/v8010009. Viruses. 2016. PMID: 26751469 Free PMC article. Review.
-
Oncolytic viruses and histone deacetylase inhibitors--a multi-pronged strategy to target tumor cells.Cytokine Growth Factor Rev. 2010 Apr-Jun;21(2-3):153-9. doi: 10.1016/j.cytogfr.2010.03.002. Epub 2010 Apr 14. Cytokine Growth Factor Rev. 2010. PMID: 20395162 Review.
Cited by
-
Oncolytic Virotherapy in Solid Tumors: The Challenges and Achievements.Cancers (Basel). 2021 Feb 3;13(4):588. doi: 10.3390/cancers13040588. Cancers (Basel). 2021. PMID: 33546172 Free PMC article. Review.
-
HDAC inhibition delays photoreceptor loss in Pde6b mutant mice of retinitis pigmentosa: insights from scRNA-seq and CUT&Tag.PeerJ. 2023 Jul 12;11:e15659. doi: 10.7717/peerj.15659. eCollection 2023. PeerJ. 2023. PMID: 37456870 Free PMC article.
-
DNA methyltransferase inhibitor 5-azacytidine enhances neuroblastoma cell lysis by an oncolytic parainfluenza virus.Anticancer Drugs. 2023 Sep 1;34(8):916-928. doi: 10.1097/CAD.0000000000001525. Epub 2023 Apr 24. Anticancer Drugs. 2023. PMID: 37227036 Free PMC article.
-
Oncolytic parainfluenza virus combines with NK cells to mediate killing of infected and non-infected lung cancer cells within 3D spheroids: role of type I and type III interferon signaling.J Immunother Cancer. 2021 Jun;9(6):e002373. doi: 10.1136/jitc-2021-002373. J Immunother Cancer. 2021. PMID: 34172515 Free PMC article.
-
Evolving Role of Oncolytic Virotherapy: Challenges and Prospects in Clinical Practice.JCO Precis Oncol. 2021 Feb 24;5:PO.20.00395. doi: 10.1200/PO.20.00395. eCollection 2021. JCO Precis Oncol. 2021. PMID: 34250386 Free PMC article. Review.
References
-
- Kinoh H., Inoue M., Washizawa K., Yamamoto T., Fujikawa S., Tokusumi Y., Iida A., Nagai Y., Hasegawa M. Generation of a recombinant Sendai virus that is selectively activated and lyses human tumor cells expressing matrix metalloproteinases. Gene Ther. 2004;11:1137–1145. doi: 10.1038/sj.gt.3302272. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

