Die in pieces: How Drosophila sheds light on neurite degeneration and clearance
- PMID: 31080046
- PMCID: PMC6541534
- DOI: 10.1016/j.jgg.2019.03.010
Die in pieces: How Drosophila sheds light on neurite degeneration and clearance
Abstract
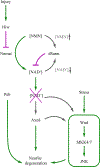
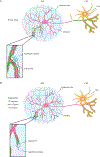
Dendrites and axons are delicate neuronal membrane extensions that undergo degeneration after physical injuries. In neurodegenerative diseases, they often degenerate prior to neuronal death. Understanding the mechanisms of neurite degeneration has been an intense focus of neurobiology research in the last two decades. As a result, many discoveries have been made in the molecular pathways that lead to neurite degeneration and the cell-cell interactions responsible for the subsequent clearance of neuronal debris. Drosophila melanogaster has served as a prime in vivo model system for identifying and characterizing the key molecular players in neurite degeneration, thanks to its genetic tractability and easy access to its nervous system. The knowledge learned in the fly provided targets and fuel for studies in other model systems that have further enhanced our understanding of neurodegeneration. In this review, we will introduce the experimental systems developed in Drosophila to investigate injury-induced neurite degeneration, and then discuss the biological pathways that drive degeneration. We will also cover what is known about the mechanisms of how phagocytes recognize and clear degenerating neurites, and how recent findings in this area enhance our understanding of neurodegenerative disease pathology.
Keywords: Axon; Dendrite; Drosophila; Injury assay; Neurite degeneration; PS exposure; Phagocyte; Phagocytosis; Wallerian degeneration.
Copyright © 2019 Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, and Genetics Society of China. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Phagocytosis and self-destruction break down dendrites of Drosophila sensory neurons at distinct steps of Wallerian degeneration.Proc Natl Acad Sci U S A. 2022 Jan 25;119(4):e2111818119. doi: 10.1073/pnas.2111818119. Proc Natl Acad Sci U S A. 2022. PMID: 35058357 Free PMC article.
-
Phosphatidylserine Externalization Results from and Causes Neurite Degeneration in Drosophila.Cell Rep. 2018 Aug 28;24(9):2273-2286. doi: 10.1016/j.celrep.2018.07.095. Cell Rep. 2018. PMID: 30157423 Free PMC article.
-
Drosophila models of neuronal injury.ILAR J. 2014;54(3):291-5. doi: 10.1093/ilar/ilt057. ILAR J. 2014. PMID: 24615442 Free PMC article. Review.
-
A photo-switchable assay system for dendrite degeneration and repair in Drosophila melanogaster.Proc Natl Acad Sci U S A. 2022 Aug 23;119(34):e2204577119. doi: 10.1073/pnas.2204577119. Epub 2022 Aug 15. Proc Natl Acad Sci U S A. 2022. PMID: 35969739 Free PMC article.
-
The Drama of Wallerian Degeneration: The Cast, Crew, and Script.Annu Rev Genet. 2021 Nov 23;55:93-113. doi: 10.1146/annurev-genet-071819-103917. Epub 2021 Aug 5. Annu Rev Genet. 2021. PMID: 34351802 Review.
Cited by
-
Elucidating the complex organization of neural micro-domains in the locust Schistocerca gregaria using dMRI.Sci Rep. 2021 Feb 9;11(1):3418. doi: 10.1038/s41598-021-82187-3. Sci Rep. 2021. PMID: 33564031 Free PMC article.
-
Local translatome sustains synaptic function in impaired Wallerian degeneration.EMBO Rep. 2025 Jan;26(1):61-83. doi: 10.1038/s44319-024-00301-8. Epub 2024 Oct 31. EMBO Rep. 2025. PMID: 39482489 Free PMC article.
-
Targeting the programmed axon degeneration pathway as a potential therapeutic for Charcot-Marie-Tooth disease.Brain Res. 2020 Jan 15;1727:146539. doi: 10.1016/j.brainres.2019.146539. Epub 2019 Nov 2. Brain Res. 2020. PMID: 31689415 Free PMC article. Review.
-
LC3-associated phagocytosis promotes glial degradation of axon debris after injury in Drosophila models.Nat Commun. 2023 May 29;14(1):3077. doi: 10.1038/s41467-023-38755-4. Nat Commun. 2023. PMID: 37248218 Free PMC article.
-
Phagocytosis and self-destruction break down dendrites of Drosophila sensory neurons at distinct steps of Wallerian degeneration.Proc Natl Acad Sci U S A. 2022 Jan 25;119(4):e2111818119. doi: 10.1073/pnas.2111818119. Proc Natl Acad Sci U S A. 2022. PMID: 35058357 Free PMC article.
References
-
- Andersen MH, Graversen H, Fedosov SN, Petersen TE, and Rasmussen JT, 2000. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry 39, 6200–6206. - PubMed
-
- Araki T, Sasaki Y, and Milbrandt J, 2004. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013. - PubMed
-
- Awasaki T, and Ito K, 2004. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr. Biol 14, 668–677. - PubMed
-
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, and Ito K, 2006. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron 50, 855–867. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

