Normalisation of circulating adiponectin levels in obese pregnant mice prevents cardiac dysfunction in adult offspring
- PMID: 31076636
- PMCID: PMC6842414
- DOI: 10.1038/s41366-019-0374-4
Normalisation of circulating adiponectin levels in obese pregnant mice prevents cardiac dysfunction in adult offspring
Abstract
Background/objectives: Adiponectin concentrations are low in obese pregnant women. Restoring normal adiponectin concentrations by infusion in obese pregnant mice prevents placental dysfunction, foetal overgrowth and metabolic syndrome in the offspring. We hypothesised that normalising maternal adiponectin in obese late pregnant dams prevents cardiac dysfunction in the adult offspring.
Subjects/methods: Pregnant female mice with diet-induced obesity were infused with adiponectin (0.62 μg g-1 day-1, n = 24) or saline (n = 22) over days 14.5-18.5 of pregnancy (term = day 19.5). Control dams ate standard chow and received saline (n = 22). Offspring were studied at 3 and 6 months of age.
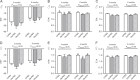
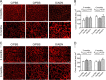
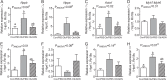
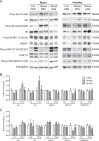
Results: Maternal obesity impaired ventricular diastolic function, increased cardiomyocyte cross-sectional area and upregulated cardiac brain natriuretic peptide (Nppb) and α-skeletal actin (Acta1) gene expression in adult male offspring, compared to control offspring. In adult female offspring, maternal obesity increased Nppb expression, decreased end-diastolic volume and caused age-dependent diastolic dysfunction but not cardiomyocyte hypertrophy. Maternal obesity also activated cardiac Akt and mechanistic target of rapamycin (mTOR) signalling in male, but not in female, offspring and inhibited cardiac extracellular signal-regulated kinase 1/2 (ERK1/2) in both sexes. Normalising maternal circulating adiponectin concentrations by infusing obese dams with adiponectin prevented offspring diastolic dysfunction and ventricular dilation and normalised cardiac Akt-mTOR signalling irrespective of sex. Maternal adiponectin infusion also reduced cardiac Nppb expression and increased ERK1/2 signalling in offspring of obese dams. Adiponectin infusion did not prevent cardiomyocyte hypertrophy but reduced ventricular wall thickness in male offspring and increased collagen content in female offspring of obese dams, compared to controls.
Conclusions: Low maternal adiponectin levels in obese mice in late pregnancy are mechanistically linked to in utero programming of cardiac dysfunction in their offspring. Interventions enhancing endogenous adiponectin secretion or signalling in obese pregnant women could prevent the development of cardiac dysfunction in their children.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
N-acetylcysteine protects neonatal mice from ventricular hypertrophy induced by maternal obesity in a sex-specific manner.Biomed Pharmacother. 2021 Jan;133:110989. doi: 10.1016/j.biopha.2020.110989. Epub 2020 Dec 8. Biomed Pharmacother. 2021. PMID: 33378994
-
Normalization of maternal adiponectin in obese pregnant mice prevents programming of impaired glucose metabolism in adult offspring.FASEB J. 2022 Jul;36(7):e22383. doi: 10.1096/fj.202200326R. FASEB J. 2022. PMID: 35670755 Free PMC article.
-
Maternal obesity causes fetal cardiac hypertrophy and alters adult offspring myocardial metabolism in mice.J Physiol. 2022 Jul;600(13):3169-3191. doi: 10.1113/JP282462. Epub 2022 May 11. J Physiol. 2022. PMID: 35545608
-
Normalizing adiponectin levels in obese pregnant mice prevents adverse metabolic outcomes in offspring.FASEB J. 2019 Feb;33(2):2899-2909. doi: 10.1096/fj.201801015R. Epub 2018 Oct 22. FASEB J. 2019. PMID: 30346829 Free PMC article.
-
Maternal high-fat diet induces long-term obesity with sex-dependent metabolic programming of adipocyte differentiation, hypertrophy and dysfunction in the offspring.Clin Sci (Lond). 2020 Apr 17;134(7):921-939. doi: 10.1042/CS20191229. Clin Sci (Lond). 2020. PMID: 32239178
Cited by
-
Clinical improvement may not reflect metabolic homeostasis normalization in subjects with and without Roux-En-Y bariatric surgery after 12 years: comparison of surgical subjects to a lean cohort.Front Endocrinol (Lausanne). 2023 Sep 21;14:1228853. doi: 10.3389/fendo.2023.1228853. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37810875 Free PMC article.
-
Insulin Increases Adipose Adiponectin in Pregnancy by Inhibiting Ubiquitination and Degradation: Impact of Obesity.J Clin Endocrinol Metab. 2022 Jan 1;107(1):53-66. doi: 10.1210/clinem/dgab680. J Clin Endocrinol Metab. 2022. PMID: 34519830 Free PMC article.
-
Maternal obesity: influencing the heart right from the start.J Physiol. 2022 Jul;600(13):3007-3008. doi: 10.1113/JP283190. Epub 2022 Jun 7. J Physiol. 2022. PMID: 35661359 Free PMC article. No abstract available.
-
Maternal obesity alters the placental transcriptome in a fetal sex-dependent manner.Front Cell Dev Biol. 2023 Jun 15;11:1178533. doi: 10.3389/fcell.2023.1178533. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37397247 Free PMC article.
-
Maternal High-Fat-High-Carbohydrate Diet-Induced Obesity Is Associated with Increased Appetite in Peripubertal Male but Not Female C57Bl/6J Mice.Nutrients. 2020 Sep 24;12(10):2919. doi: 10.3390/nu12102919. Nutrients. 2020. PMID: 32987812 Free PMC article.
References
-
- Branum AM, Kirmeyer SE, Gregory EC. Prepregnancy body mass index by maternal characteristics and state: data from the birth certificate, 2014. Natl Vital Stat Rep. 2016;65:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

