Real-world safety and efficacy of paritaprevir/ritonavir/ombitasvir plus dasabuvir ± ribavirin in patients with hepatitis C virus genotype 1 and advanced hepatic fibrosis or compensated cirrhosis: a multicenter pooled analysis
- PMID: 31068655
- PMCID: PMC6506536
- DOI: 10.1038/s41598-019-43554-3
Real-world safety and efficacy of paritaprevir/ritonavir/ombitasvir plus dasabuvir ± ribavirin in patients with hepatitis C virus genotype 1 and advanced hepatic fibrosis or compensated cirrhosis: a multicenter pooled analysis
Abstract
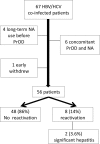
Paritaprevir/ritonavir, ombitasvir, and dasabuvir (PrOD) with or without ribavirin shows favorable results in hepatitis C virus genotype 1 (HCV-1) patients in terms of safety and efficacy, but real-world data remain limited for those with advanced hepatic fibrosis (fibrosis 3, F3) or compensated cirrhosis (F4). A total of 941 patients treated in four hospitals (the Keelung, the Linkuo, the Chiayi and the Kaohsiung Chang Gung Memorial Hospital) through a nationwide government-funded program in Taiwan were enrolled. Patients with HCV and advanced hepatic fibrosis or compensated cirrhosis received 12 weeks of PrOD in HCV-1b and 12 or 24 weeks of PrOD plus ribavirin therapy in HCV-1a without or with cirrhosis. Advanced hepatic fibrosis or compensated cirrhosis was confirmed by either ultrasonography, fibrosis index based on 4 factors (FIB-4) test, or transient elastography/acoustic radiation force impulse (ARFI). The safety and efficacy (sustained virologic response 12 weeks off therapy, SVR12) were evaluated. An SVR12 was achieved in 887 of 898 (98.8%) patients based on the per-protocol analysis (subjects receiving ≥1 dose of any study medication and HCV RNA data available at post-treatment week 12). Child-Pugh A6 (odds ratio: 0.168; 95% confidence interval (CI): 0.043-0.659, p = 0.011) was the only significant factor of poor SVR12. Fifty-four (5.7%) patients were withdrawn early from the treatment because of hepatic decompensation (n = 18, 1.9%) and other adverse reactions. Multivariate analyses identified old age (odds ratio: 1.062; 95% CI: 1.008-1.119, p = 0.024) and Child-Pugh A6 (odds ratio: 4.957; 95% CI: 1.691-14.528, p = 0.004) were significantly associated with hepatic decompensation. In conclusion, this large real-world cohort proved PrOD with or without ribavirin to be highly effective in chronic hepatitis C patients with advanced hepatic fibrosis or compensated cirrhosis. However, Child-Pugh A6 should be an exclusion criterion for first-line treatment in these patients.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Ombitasvir, paritaprevir, and ritonavir, with or without dasabuvir, plus ribavirin for patients with hepatitis C virus genotype 1 or 4 infection with cirrhosis (ABACUS): a prospective observational study.Lancet Gastroenterol Hepatol. 2017 Jun;2(6):427-434. doi: 10.1016/S2468-1253(17)30048-1. Epub 2017 Apr 10. Lancet Gastroenterol Hepatol. 2017. PMID: 28497758
-
Paritaprevir/ritonavir/ombitasvir plus dasabuvir with ribavirin for treatment of recurrent chronic hepatitis C genotype 1 infection after liver transplantation: Real-world experience.J Formos Med Assoc. 2018 Jun;117(6):518-526. doi: 10.1016/j.jfma.2017.06.006. Epub 2017 Jun 26. J Formos Med Assoc. 2018. PMID: 28662883
-
Ombitasvir/paritaprevir/ritonavir+dasabuvir+ribavirin for chronic hepatitis C virus genotype 1b-infected cirrhotics (TURQUOISE-IV).Eur J Gastroenterol Hepatol. 2018 Sep;30(9):1073-1076. doi: 10.1097/MEG.0000000000001166. Eur J Gastroenterol Hepatol. 2018. PMID: 29762255 Clinical Trial.
-
Ombitasvir/paritaprevir/ritonavir and dasabuvir tablets for hepatitis C virus genotype 1 infection.Ann Pharmacother. 2015 May;49(5):566-81. doi: 10.1177/1060028015570729. Epub 2015 Feb 13. Ann Pharmacother. 2015. PMID: 25680759 Review.
-
Profile of paritaprevir/ritonavir/ombitasvir plus dasabuvir in the treatment of chronic hepatitis C virus genotype 1 infection.Drug Des Devel Ther. 2015 Nov 13;9:6083-94. doi: 10.2147/DDDT.S80226. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26622169 Free PMC article. Review.
Cited by
-
Factors associated with treatment failure of direct-acting antivirals for chronic hepatitis C: A real-world nationwide hepatitis C virus registry programme in Taiwan.Liver Int. 2021 Jun;41(6):1265-1277. doi: 10.1111/liv.14849. Epub 2021 Mar 12. Liver Int. 2021. PMID: 33655714 Free PMC article.
-
Glycemic Control in Patients Undergoing Treatment With Paritaprevir/Ombitasvir/Ritonavir and Dasabuvir for Chronic Hepatitis C Infection.In Vivo. 2022 May-Jun;36(3):1438-1443. doi: 10.21873/invivo.12849. In Vivo. 2022. PMID: 35478152 Free PMC article.
-
Treatment effectiveness and side effects of patients with hepatitis C in the prisons of Southern Taiwan: a real-life retrospective analysis.BMJ Open. 2023 Jun 7;13(6):e070490. doi: 10.1136/bmjopen-2022-070490. BMJ Open. 2023. PMID: 37286314 Free PMC article.
-
Clinical utility of hepatitis C virus core antigen assay in the monitoring of direct-acting antivirals for chronic hepatitis C.PLoS One. 2020 Mar 3;15(3):e0229994. doi: 10.1371/journal.pone.0229994. eCollection 2020. PLoS One. 2020. PMID: 32126125 Free PMC article.
-
Viral proteases as therapeutic targets.Mol Aspects Med. 2022 Dec;88:101159. doi: 10.1016/j.mam.2022.101159. Epub 2022 Nov 29. Mol Aspects Med. 2022. PMID: 36459838 Free PMC article. Review.
References
-
- World Health Organization. Hepatitis C. WHO fact sheet 164. Available at, http://www.who.int/mediacentre/factsheets/fs164/en (2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

