Hybrids made from antimicrobial peptides with different mechanisms of action show enhanced membrane permeabilization
- PMID: 31067436
- PMCID: PMC6721965
- DOI: 10.1016/j.bbamem.2019.05.002
Hybrids made from antimicrobial peptides with different mechanisms of action show enhanced membrane permeabilization
Abstract
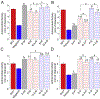
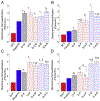
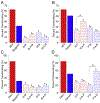
Combining two known antimicrobial peptides (AMPs) into a hybrid peptide is one promising avenue in the design of agents with increased antibacterial activity. However, very few previous studies have considered the effect of creating a hybrid from one AMP that permeabilizes membranes and another AMP that acts intracellularly after translocating across the membrane. Moreover, very few studies have systematically evaluated the order of parent peptides or the presence of linkers in the design of hybrid AMPs. Here, we use a combination of antibacterial measurements, cellular assays and semi-quantitative confocal microscopy to characterize the activity and mechanism for a library of sixteen hybrid peptides. These hybrids consist of permutations of two primarily membrane translocating peptides, buforin II and DesHDAP1, and two primarily membrane permeabilizing peptides, magainin 2 and parasin. For all hybrids, the permeabilizing peptide appeared to dominate the mechanism, with hybrids primarily killing bacteria through membrane permeabilization. We also observed increased hybrid activity when the permeabilizing parent peptide was placed at the N-terminus. Activity data also highlighted the potential value of considering AMP cocktails in addition to hybrid peptides. Together, these observations will guide future design efforts aiming to design more active hybrid AMPs.
Keywords: Antimicrobial peptide; Hybrid peptide; Membrane permeabilization; Membrane translocation; Peptide design.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Activity and characterization of a pH-sensitive antimicrobial peptide.Biochim Biophys Acta Biomembr. 2019 Oct 1;1861(10):182984. doi: 10.1016/j.bbamem.2019.05.006. Epub 2019 May 8. Biochim Biophys Acta Biomembr. 2019. PMID: 31075228 Free PMC article.
-
Modular analysis of hipposin, a histone-derived antimicrobial peptide consisting of membrane translocating and membrane permeabilizing fragments.Biochim Biophys Acta. 2014 Sep;1838(9):2228-2233. doi: 10.1016/j.bbamem.2014.04.010. Epub 2014 Apr 18. Biochim Biophys Acta. 2014. PMID: 24747525 Free PMC article.
-
Beyond electrostatics: Antimicrobial peptide selectivity and the influence of cholesterol-mediated fluidity and lipid chain length on protegrin-1 activity.Biochim Biophys Acta Biomembr. 2019 Oct 1;1861(10):182977. doi: 10.1016/j.bbamem.2019.04.011. Epub 2019 May 8. Biochim Biophys Acta Biomembr. 2019. PMID: 31077677
-
Magainins as paradigm for the mode of action of pore forming polypeptides.Biochim Biophys Acta. 1998 Nov 10;1376(3):391-400. doi: 10.1016/s0304-4157(98)00014-8. Biochim Biophys Acta. 1998. PMID: 9804997 Review.
-
Solution NMR studies of amphibian antimicrobial peptides: linking structure to function?Biochim Biophys Acta. 2009 Aug;1788(8):1639-55. doi: 10.1016/j.bbamem.2009.01.002. Epub 2009 Jan 15. Biochim Biophys Acta. 2009. PMID: 19272309 Review.
Cited by
-
A Bifunctional Peptide Conjugate That Controls Infections of Erwinia amylovora in Pear Plants.Molecules. 2021 Jun 5;26(11):3426. doi: 10.3390/molecules26113426. Molecules. 2021. PMID: 34198776 Free PMC article.
-
Peptide Conjugates Derived from flg15, Pep13, and PIP1 That Are Active against Plant-Pathogenic Bacteria and Trigger Plant Defense Responses.Appl Environ Microbiol. 2022 Jun 28;88(12):e0057422. doi: 10.1128/aem.00574-22. Epub 2022 May 31. Appl Environ Microbiol. 2022. PMID: 35638842 Free PMC article.
-
Rational Designed Hybrid Peptides Show up to a 6-Fold Increase in Antimicrobial Activity and Demonstrate Different Ultrastructural Changes as the Parental Peptides Measured by BioSAXS.Front Pharmacol. 2021 Dec 3;12:769739. doi: 10.3389/fphar.2021.769739. eCollection 2021. Front Pharmacol. 2021. PMID: 34966279 Free PMC article.
-
Extracellular Polymeric Substance Protects Some Cells in an Escherichia coli Biofilm from the Biomechanical Consequences of Treatment with Magainin 2.Microorganisms. 2021 Apr 30;9(5):976. doi: 10.3390/microorganisms9050976. Microorganisms. 2021. PMID: 33946431 Free PMC article.
-
Nanosystems as Vehicles for the Delivery of Antimicrobial Peptides (AMPs).Pharmaceutics. 2019 Sep 2;11(9):448. doi: 10.3390/pharmaceutics11090448. Pharmaceutics. 2019. PMID: 31480680 Free PMC article. Review.
References
-
- Zasloff M, Antimicrobial peptides of multicellular organisms, Nature, 415 (2000) 389–395. - PubMed
-
- Koo YS, Kim JM, Park IY, Yu BJ, Jang SA, Kim KS, Park CB, Cho JH, Kim SC, Structure–activity relations of parasin I, a histone H2A-derived antimicrobial peptide, Peptides, 29 (2008) 1102–1108. - PubMed
-
- Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW, Membrane pores induced byMagainin, Biochemistry, 35 (1996) 13723–13728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

