A Review of the Therapeutic Potential of Recently Developed G Protein-Biased Kappa Agonists
- PMID: 31057409
- PMCID: PMC6478756
- DOI: 10.3389/fphar.2019.00407
A Review of the Therapeutic Potential of Recently Developed G Protein-Biased Kappa Agonists
Abstract
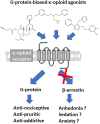
Between 2000 and 2005 several studies revealed that morphine is more potent and exhibits fewer side effects in beta-arrestin 2 knockout mice. These findings spurred efforts to develop opioids that signal primarily via G protein activation and do not, or only very weakly, recruit beta-arrestin. Development of such molecules targeting the mu opioid receptor initially outpaced those targeting the kappa, delta and nociceptin opioid receptors, with the G protein-biased mu opioid agonist oliceridine/TRV130 having completed phase III clinical trials with improved therapeutic window to treat moderate-to-severe acute pain. Recently however, there has been a sharp increase in the development of novel G protein-biased kappa agonists. It is hypothesized that G protein-biased kappa agonists can reduce pain and itch, but exhibit fewer side effects, such as anhedonia and psychosis, that have thus far limited the clinical development of unbiased kappa opioid agonists. Here we summarize recently discovered G protein-biased kappa agonists, comparing structures, degree of signal bias and preclinical effects. We specifically reviewed nalfurafine, 22-thiocyanatosalvinorin A (RB-64), mesyl-salvinorin B, 2-(4-(furan-2-ylmethyl)-5-((4-methyl-3-(trifluoromethyl)benzyl)thio)-4H-1,2,4-triazol-3-yl)pyridine (triazole 1.1), 3-(2-((cyclopropylmethyl)(phenethyl)amino)ethyl)phenol (HS666), N-n-butyl-N-phenylethyl-N-3-hydroxyphenylethyl-amine (compound 5/BPHA), 6-guanidinonaltrindole (6'GNTI), and collybolide. These agonists encompass a variety of chemical scaffolds and range in both their potency and efficacy in terms of G protein signaling and beta-arrestin recruitment. Thus unsurprisingly, the behavioral responses reported for these agonists are not uniform. Yet, it is our conclusion that the kappa opioid field will benefit tremendously from future studies that compare several biased agonists and correlate the degree of signaling bias to a particular pharmacological response.
Keywords: G protein; antinociception; beta-arrestin; diphenethylamine; kappa opioid receptor; nalfurafine; side effects; signaling bias.
Figures

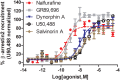
 ), GR89,696 (n = 4,
), GR89,696 (n = 4,  ), dynorphin A (n = 4, blue
), dynorphin A (n = 4, blue  ), U50,488 (n = 4, ) and salvinorin A (n = 4, brown
), U50,488 (n = 4, ) and salvinorin A (n = 4, brown  ) in PathHunter U2OS hOPRK1/β-arrestin-2 cells (DiscoverX, Fremont, CA, United States). Each concentration was tested in duplicate, and three independent dose-response curves were produced for each agonist as previously described (Chiang et al., 2016). Data is normalized to U50,488. Nalfurafine was purchased from AdooQ (Irvine, CA, United States) all other compounds were from Tocris (Minneapolis, MN, United States).
) in PathHunter U2OS hOPRK1/β-arrestin-2 cells (DiscoverX, Fremont, CA, United States). Each concentration was tested in duplicate, and three independent dose-response curves were produced for each agonist as previously described (Chiang et al., 2016). Data is normalized to U50,488. Nalfurafine was purchased from AdooQ (Irvine, CA, United States) all other compounds were from Tocris (Minneapolis, MN, United States).
 , G-protein) and EC50 potency for β-arrestin 2 recruitment of 550 nM and 100% efficacy (solid line, light blue
, G-protein) and EC50 potency for β-arrestin 2 recruitment of 550 nM and 100% efficacy (solid line, light blue  ). In contrast an ‘efficacy-dominant” κOR agonist, that resembles HS666 with an EC50 potency for β-arrestin 2 recruitment of 550 nM, but 10% efficacy (solid line, blue
). In contrast an ‘efficacy-dominant” κOR agonist, that resembles HS666 with an EC50 potency for β-arrestin 2 recruitment of 550 nM, but 10% efficacy (solid line, blue  ), only weakly recruits β-arrestin 2 even at high concentrations (top right panel). Consider the endogenous agonist dynorphin A, which recruits β-arrestin 2 at 100% efficacy (middle panel). At high concentrations the affinity-dominant agonist will displace dynorphin from the κOR, yet retain highly efficacious recruitment of β-arrestin 2 (bottom left panel). In contrast the efficacy-dominant agonist will reduce β-arrestin 2 recruitment efficacy once this type of agonists displaces dynorphin from the κOR (bottom right) panel.
), only weakly recruits β-arrestin 2 even at high concentrations (top right panel). Consider the endogenous agonist dynorphin A, which recruits β-arrestin 2 at 100% efficacy (middle panel). At high concentrations the affinity-dominant agonist will displace dynorphin from the κOR, yet retain highly efficacious recruitment of β-arrestin 2 (bottom left panel). In contrast the efficacy-dominant agonist will reduce β-arrestin 2 recruitment efficacy once this type of agonists displaces dynorphin from the κOR (bottom right) panel.Similar articles
-
Structurally Related Kappa Opioid Receptor Agonists with Substantial Differential Signaling Bias: Neuroendocrine and Behavioral Effects in C57BL6 Mice.Int J Neuropsychopharmacol. 2018 Sep 1;21(9):847-857. doi: 10.1093/ijnp/pyy034. Int J Neuropsychopharmacol. 2018. PMID: 29635340 Free PMC article.
-
Characterization of structurally novel G protein biased CB1 agonists: Implications for drug development.Pharmacol Res. 2017 Nov;125(Pt B):161-177. doi: 10.1016/j.phrs.2017.08.008. Epub 2017 Aug 23. Pharmacol Res. 2017. PMID: 28838808 Free PMC article.
-
Biased versus Partial Agonism in the Search for Safer Opioid Analgesics.Molecules. 2020 Aug 25;25(17):3870. doi: 10.3390/molecules25173870. Molecules. 2020. PMID: 32854452 Free PMC article. Review.
-
An updated assessment of the translational promise of G-protein-biased kappa opioid receptor agonists to treat pain and other indications without debilitating adverse effects.Pharmacol Res. 2022 Mar;177:106091. doi: 10.1016/j.phrs.2022.106091. Epub 2022 Jan 29. Pharmacol Res. 2022. PMID: 35101565 Free PMC article. Review.
-
Nalfurafine is a G-protein biased agonist having significantly greater bias at the human than rodent form of the kappa opioid receptor.Cell Signal. 2017 Apr;32:59-65. doi: 10.1016/j.cellsig.2017.01.016. Epub 2017 Jan 11. Cell Signal. 2017. PMID: 28088389 Free PMC article.
Cited by
-
Strategies for Developing κ Opioid Receptor Agonists for the Treatment of Pain with Fewer Side Effects.J Pharmacol Exp Ther. 2020 Nov;375(2):332-348. doi: 10.1124/jpet.120.000134. Epub 2020 Sep 10. J Pharmacol Exp Ther. 2020. PMID: 32913006 Free PMC article. Review.
-
Synthesis and evaluation of 3,4,5-trisubstituted triazoles as G protein-biased kappa opioid receptor agonists.Eur J Med Chem. 2024 Oct 5;276:116627. doi: 10.1016/j.ejmech.2024.116627. Epub 2024 Jun 27. Eur J Med Chem. 2024. PMID: 38971050
-
Influence of G protein-biased agonists of μ-opioid receptor on addiction-related behaviors.Pharmacol Rep. 2021 Aug;73(4):1033-1051. doi: 10.1007/s43440-021-00251-1. Epub 2021 Apr 9. Pharmacol Rep. 2021. PMID: 33835467 Free PMC article. Review.
-
Pharmacology of Kappa Opioid Receptors: Novel Assays and Ligands.Front Pharmacol. 2022 Apr 21;13:873082. doi: 10.3389/fphar.2022.873082. eCollection 2022. Front Pharmacol. 2022. PMID: 35529436 Free PMC article.
-
Sex- and β-arrestin-dependent effects of kappa opioid receptor-mediated ethanol consumption.Pharmacol Biochem Behav. 2022 May;216:173377. doi: 10.1016/j.pbb.2022.173377. Epub 2022 Mar 29. Pharmacol Biochem Behav. 2022. PMID: 35364122 Free PMC article.
References
-
- Allen J. A., Yost J. M., Setola V., Chen X., Sassano M. F., Chen M., et al. (2011). Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. U.S.A. 108 18488–18493. 10.1073/pnas.1104807108 - DOI - PMC - PubMed
-
- Altarifi A. A., David B., Muchhala K. H., Blough B. E., Akbarali H., Negus S. S. (2017). Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J. Psychopharmacol. 31 730–739. 10.1177/0269881116689257 - DOI - PMC - PubMed
-
- Austin Zamarripa C., Edwards S. R., Qureshi H. N., Yi J. N., Blough B. E., Freeman K. B. (2018). The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol. Depend. 192 158–162. 10.1016/j.drugalcdep.2018.08.002 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

