Development of an E2 ELISA Methodology to Assess Chikungunya Seroprevalence in Patients from an Endemic Region of Mexico
- PMID: 31052472
- PMCID: PMC6563309
- DOI: 10.3390/v11050407
Development of an E2 ELISA Methodology to Assess Chikungunya Seroprevalence in Patients from an Endemic Region of Mexico
Abstract
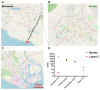
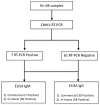
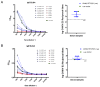
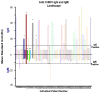
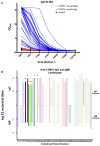
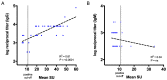
Chikungunya fever is a debilitating disease caused by Chikungunya virus (CHIKV) that can result in long-lasting arthralgias. The early diagnosis of CHIKV relies on PCR during the acute infection phase to allow differential diagnosis with other co-circulating arboviruses such as dengue and Zika. Alternatively, serology can support diagnosis and provide epidemiological information on current and past outbreaks. Many commercial serological ELISA assays are based on the inactivated whole CHIKV, but their sensitivity and specificity show great variability. We produced recombinant CHIKV E2 that is suitable for ELISA assays, which was used for the serodiagnosis of CHIKV infections occurring in an arbovirus endemic Mexican region within Michoacán state. A cross-sectional study was conducted in 2016-2017; sera was obtained from 15 healthy donors and 68 patients presenting undifferentiated febrile illness. Serum samples were screened by RT-PCR and by our in-house ELISA assay. Our results indicate that IgM and IgG anti-CHIKV E2 antibodies were detected with our ELISA assay with higher sensitivity than a commercially available CHIKV ELISA kit. Our simple and sensitive ELISA assay for the serodiagnosis of CHIKV infections can be applied to population-based seroprevalence surveys and has potential for monitoring vaccine immunogenicity in CHIKV vaccine clinical trials.
Keywords: CHIKV antibodies; Chikungunya virus; ELISA; Mexico; diagnosis; envelope protein 2; febrile patients.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Enzyme-linked immunosorbent assay using recombinant envelope protein 2 antigen for diagnosis of Chikungunya virus.Virol J. 2018 Jul 24;15(1):112. doi: 10.1186/s12985-018-1028-1. Virol J. 2018. PMID: 30041676 Free PMC article.
-
Evaluation of an immunochromatography rapid diagnosis kit for detection of chikungunya virus antigen in India, a dengue-endemic country.Virol J. 2018 May 11;15(1):84. doi: 10.1186/s12985-018-1000-0. Virol J. 2018. PMID: 29751761 Free PMC article.
-
Development and evaluation of recombinant E2 protein based IgM capture enzyme-linked immunosorbent assay (ELISA) and double antigen sandwich ELISA for detection of antibodies to Chikungunya virus.PLoS Negl Trop Dis. 2022 Dec 8;16(12):e0010829. doi: 10.1371/journal.pntd.0010829. eCollection 2022 Dec. PLoS Negl Trop Dis. 2022. PMID: 36480572 Free PMC article.
-
Luciferase Immunosorbent Assay Based on Multiple E Antigens for the Detection of Chikungunya Virus-Specific IgG Antibodies.Microbiol Spectr. 2022 Apr 27;10(2):e0149621. doi: 10.1128/spectrum.01496-21. Epub 2022 Mar 21. Microbiol Spectr. 2022. PMID: 35311573 Free PMC article. Review.
-
Evidence of previous but not current transmission of chikungunya virus in southern and central Vietnam: Results from a systematic review and a seroprevalence study in four locations.PLoS Negl Trop Dis. 2018 Feb 9;12(2):e0006246. doi: 10.1371/journal.pntd.0006246. eCollection 2018 Feb. PLoS Negl Trop Dis. 2018. PMID: 29425199 Free PMC article. Review.
Cited by
-
Understanding the Biology and Immune Pathogenesis of Chikungunya Virus Infection for Diagnostic and Vaccine Development.Viruses. 2022 Dec 23;15(1):48. doi: 10.3390/v15010048. Viruses. 2022. PMID: 36680088 Free PMC article. Review.
-
Evidence of Chikungunya virus seroprevalence in Myanmar among dengue-suspected patients and healthy volunteers in 2013, 2015, and 2018.PLoS Negl Trop Dis. 2021 Dec 1;15(12):e0009961. doi: 10.1371/journal.pntd.0009961. eCollection 2021 Dec. PLoS Negl Trop Dis. 2021. PMID: 34851949 Free PMC article.
-
Chikungunya E2 Protein Produced in E. coli and HEK293-T Cells-Comparison of Their Performances in ELISA.Viruses. 2020 Aug 26;12(9):939. doi: 10.3390/v12090939. Viruses. 2020. PMID: 32858804 Free PMC article.
-
Chikungunya Virus and (Re-) Emerging Alphaviruses.Viruses. 2019 Aug 24;11(9):779. doi: 10.3390/v11090779. Viruses. 2019. PMID: 31450552 Free PMC article.
-
Development of Highly Sensitive Sandwich ELISA for the Early-Phase Diagnosis of Chikungunya Virus Utilizing rE2-E1 Protein.Infect Drug Resist. 2022 Jul 28;15:4065-4078. doi: 10.2147/IDR.S347545. eCollection 2022. Infect Drug Resist. 2022. PMID: 35924014 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

