Exosomes in cancer development, metastasis, and immunity
- PMID: 31047959
- PMCID: PMC6542596
- DOI: 10.1016/j.bbcan.2019.04.004
Exosomes in cancer development, metastasis, and immunity
Abstract
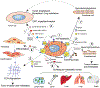
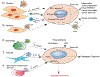
Exosomes play essential roles in intercellular communications. The exosome was discovered in 1983, when it was found that reticulocytes release 50-nm small vesicles carrying transferrin receptors into the extracellular space. Since then, our understanding of the mechanism and function of the exosome has expanded exponentially that has transformed our perspective of inter-cellular exchanges and the molecular mechanisms that underlie disease progression. Cancer cells generally produce more exosomes than normal cells, and exosomes derived from cancer cells have a strong capacity to modify both local and distant microenvironments. In this review, we summarize the functions of exosomes in cancer development, metastasis, and anti-tumor or pro-tumor immunity, plus their application in cancer treatment and diagnosis/prognosis. Although the exosome field has rapidly advanced, we still do not fully understand the regulation and function of exosomes in detail and still face many challenges in their clinical application. Continued discoveries in this field will bring novel insights on intercellular communications involved in various biological functions and disease progression, thus empowering us to effectively tackle accompanying clinical challenges.
Keywords: Cancer; Exosome; Extracellular vesicles; Immunity; Intercellular communication; Metastasis.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Exosome-Mediated Metastasis: Communication from a Distance.Dev Cell. 2019 May 6;49(3):347-360. doi: 10.1016/j.devcel.2019.04.011. Dev Cell. 2019. PMID: 31063754 Review.
-
Exosome: Function and Role in Cancer Metastasis and Drug Resistance.Technol Cancer Res Treat. 2018 Jan 1;17:1533033818763450. doi: 10.1177/1533033818763450. Technol Cancer Res Treat. 2018. PMID: 29681222 Free PMC article. Review.
-
The role of exosomes in metastasis and progression of melanoma.Cancer Treat Rev. 2020 Apr;85:101975. doi: 10.1016/j.ctrv.2020.101975. Epub 2020 Jan 22. Cancer Treat Rev. 2020. PMID: 32050108 Review.
-
Cancer-derived exosomic microRNAs shape the immune system within the tumor microenvironment: State of the art.Semin Cell Dev Biol. 2017 Jul;67:23-28. doi: 10.1016/j.semcdb.2016.12.004. Epub 2016 Dec 9. Semin Cell Dev Biol. 2017. PMID: 27956165 Free PMC article. Review.
-
Tumour-derived exosomes: Tiny envelopes for big stories.Biol Cell. 2015 Sep;107(9):287-305. doi: 10.1111/boc.201400095. Epub 2015 Jun 9. Biol Cell. 2015. PMID: 25923825 Review.
Cited by
-
Non-Coding RNAs of Extracellular Vesicles: Key Players in Organ-Specific Metastasis and Clinical Implications.Cancers (Basel). 2022 Nov 19;14(22):5693. doi: 10.3390/cancers14225693. Cancers (Basel). 2022. PMID: 36428785 Free PMC article. Review.
-
One-Step Formation Method of Plasmid DNA-Loaded, Extracellular Vesicles-Mimicking Lipid Nanoparticles Based on Nucleic Acids Dilution-Induced Assembly.Cells. 2024 Jul 11;13(14):1183. doi: 10.3390/cells13141183. Cells. 2024. PMID: 39056764 Free PMC article.
-
S100A9‑containing serum exosomes obtained from patients with burn injuries promote myocardial cell pyroptosis through NLRP3.Exp Ther Med. 2022 Sep 1;24(5):646. doi: 10.3892/etm.2022.11583. eCollection 2022 Nov. Exp Ther Med. 2022. PMID: 36277145 Free PMC article.
-
Different origin-derived exosomes and their clinical advantages in cancer therapy.Front Immunol. 2024 Jun 27;15:1401852. doi: 10.3389/fimmu.2024.1401852. eCollection 2024. Front Immunol. 2024. PMID: 38994350 Free PMC article. Review.
-
Extracellular Matrix Cues Regulate Mechanosensing and Mechanotransduction of Cancer Cells.Cells. 2024 Jan 2;13(1):96. doi: 10.3390/cells13010096. Cells. 2024. PMID: 38201302 Free PMC article. Review.
References
-
- Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, Fang J, Rampersaud S, Hoshino A, Matei I, Kenific CM, Nakajima M, Mutvei AP, Sansone P, Buehring W, Wang H, Jimenez JP, Cohen-Gould L, Paknejad N, Brendel M, Manova-Todorova K, Magalhaes A, Ferreira JA, Osorio H, Silva AM, Massey A, Cubillos-Ruiz JR, Galletti G, Giannakakou P, Cuervo AM, Blenis J, Schwartz R, Brady MS, Peinado H, Bromberg J, Matsui H, Reis CA, Lyden D, Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation, Nat Cell Biol, 20 (2018) 332–343, DOI: 10.1038/s41556-018-0040-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

