A study to assess the methodological quality of in vivo animal experiments published in Indian journal of pharmacology: A retrospective, cross-sectional, observational study
- PMID: 31031462
- PMCID: PMC6444832
- DOI: 10.4103/ijp.IJP_536_18
A study to assess the methodological quality of in vivo animal experiments published in Indian journal of pharmacology: A retrospective, cross-sectional, observational study
Abstract
Background: Good reporting of experimental research is a vital part of research process. Although the reporting guidelines such as Animal research: Reporting in vivo experiments (ARRIVE) require the authors to stick to a standard format, they do not guarantee study reports' validity. For assessing the study reports validity, critical appraisal tools are required.
Objective: The evaluation of the methodological quality of in vivo animal studies of Indian Journal of Pharmacology (IJP) published between 2011 and 2017 through critical appraisal was the primary objective. The secondary objective was to evaluate the adherence of selected studies to the ARRIVE guidelines.
Materials and methods: All in vivo animal studies published as full-text articles in IJP from January 2011 to December 2017 and satisfying the inclusion norms were included. A checklist based on the underlying principles of ARRIVE statement was applied to in vivo animal research (AR) published in IJP. For critical appraisal of reports, risk-of-bias domains were also applied on studies from in vivo AR.
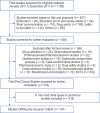
Results: One hundred and sixty-one studies satisfied the inclusion criteria. Seventy-three articles (45.34%, 95% confidence interval [CI]: 0.37-0.53) discussed about randomization procedure. Eighteen articles (11.18%, 95% CI: 0.07-0.16) gave details about blinding when assessing results. None of the studies provided details of sample size calculation. Eight articles (4.97%, 95% CI: 0.02-0.09) commented on the study limitations.
Conclusion: It was found that adherence to only some criteria of ARRIVE guidelines was subpar. There is a need for optimal reporting of random distribution of animals to experimental groups, concealment of allocation, blinded outcome evaluation, computation of sample size, and attrition of animals for improving the validity of AR.
Keywords: Animal research: reporting in vivo experiments guidelines; critical appraisal; in vivo animal research; methodological quality; risk-of-bias tool.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
A Study to Assess the Quality of Reporting of Animal Research Studies Published in PubMed Indexed Journals: A Retrospective, Cross-Sectional Content Analysis.Cureus. 2022 Jan 19;14(1):e21439. doi: 10.7759/cureus.21439. eCollection 2022 Jan. Cureus. 2022. PMID: 35198334 Free PMC article.
-
Standard of reporting animal-based experimental research in Indian Journal of Pharmacology.Indian J Pharmacol. 2015 Sep-Oct;47(5):514-7. doi: 10.4103/0253-7613.165188. Indian J Pharmacol. 2015. PMID: 26600640 Free PMC article.
-
Quality of reporting of interventional animal studies in rheumatology: a systematic review using the ARRIVE guidelines.Int J Rheum Dis. 2015 Jun;18(5):488-94. doi: 10.1111/1756-185X.12699. Int J Rheum Dis. 2015. PMID: 26082348 Review.
-
The critical appraisal of randomized controlled trials published in an Indian journal to assess the quality of reporting: A retrospective, cross-sectional study.Perspect Clin Res. 2022 Jan-Mar;13(1):33-37. doi: 10.4103/picr.PICR_169_19. Epub 2021 Jan 8. Perspect Clin Res. 2022. PMID: 35198426 Free PMC article.
-
Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research.PLoS Biol. 2010 Jun 29;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. PLoS Biol. 2010. PMID: 20613859 Free PMC article. Review. No abstract available.
Cited by
-
Intraperitoneal drug delivery systems releasing cytostatic agents to target gastro-intestinal peritoneal metastases in laboratory animals: a systematic review.Clin Exp Metastasis. 2022 Aug;39(4):541-579. doi: 10.1007/s10585-022-10173-8. Epub 2022 Jun 23. Clin Exp Metastasis. 2022. PMID: 35737252 Free PMC article. Review.
-
A Study to Assess the Quality of Reporting of Animal Research Studies Published in PubMed Indexed Journals: A Retrospective, Cross-Sectional Content Analysis.Cureus. 2022 Jan 19;14(1):e21439. doi: 10.7759/cureus.21439. eCollection 2022 Jan. Cureus. 2022. PMID: 35198334 Free PMC article.
References
-
- Steward O, Balice-Gordon R. Rigor or mortis: Best practices for preclinical research in neuroscience. Neuron. 2014;84:572–81. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials