Protein and Glycan Mimicry in HIV Vaccine Design
- PMID: 31028779
- PMCID: PMC6556556
- DOI: 10.1016/j.jmb.2019.04.016
Protein and Glycan Mimicry in HIV Vaccine Design
Abstract
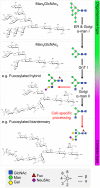
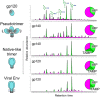
Antigenic mimicry is a fundamental tenet of structure-based vaccinology. Vaccine strategies for the human immunodeficiency virus type 1 (HIV-1) focus on the mimicry of its envelope spike (Env) due to its exposed location on the viral membrane and role in mediating infection. However, the virus has evolved to minimize the immunogenicity of conserved epitopes on the envelope spike. This principle is starkly illustrated by the presence of an extensive array of host-derived glycans, which act to shield the underlying protein from antibody recognition. Despite these hurdles, a subset of HIV-infected individuals eventually develop broadly neutralizing antibodies that recognize these virally presented glycans. Effective HIV-1 immunogens are therefore likely to involve some degree of mimicry of both the protein and glycan components of Env. As such, considerable efforts have been made to characterize the structure of the envelope spike and its glycan shield. This review summarizes the recent progress made in this field, with an emphasis on our growing understanding of the factors shaping the glycan shield of Env derived from both virus and soluble immunogens. We argue that recombinant mimics of the envelope spike are currently capable of capturing many features of the native viral glycan shield. Finally, we explore strategies through which the immunogenicity of Env glycans may be enhanced in the development of future immunogens.
Keywords: antibodies; glycosylation; human immunodeficiency virus; structure; vaccinology.
Copyright © 2019 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


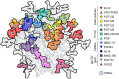


Similar articles
-
Beyond glycan barriers: non-cognate ligands and protein mimicry approaches to elicit broadly neutralizing antibodies for HIV-1.J Biomed Sci. 2024 Aug 21;31(1):83. doi: 10.1186/s12929-024-01073-y. J Biomed Sci. 2024. PMID: 39169357 Free PMC article. Review.
-
Changes in Structure and Antigenicity of HIV-1 Env Trimers Resulting from Removal of a Conserved CD4 Binding Site-Proximal Glycan.J Virol. 2016 Sep 29;90(20):9224-36. doi: 10.1128/JVI.01116-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489265 Free PMC article.
-
Limited Evidence for a Relationship between HIV-1 Glycan Shield Features in Early Infection and the Development of Neutralization Breadth.J Virol. 2021 Aug 10;95(17):e0079721. doi: 10.1128/JVI.00797-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34160251 Free PMC article.
-
Murine Antibody Responses to Cleaved Soluble HIV-1 Envelope Trimers Are Highly Restricted in Specificity.J Virol. 2015 Oct;89(20):10383-98. doi: 10.1128/JVI.01653-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246566 Free PMC article.
-
Antibody responses to the HIV-1 envelope high mannose patch.Adv Immunol. 2019;143:11-73. doi: 10.1016/bs.ai.2019.08.002. Epub 2019 Sep 11. Adv Immunol. 2019. PMID: 31607367 Free PMC article. Review.
Cited by
-
Conformational antigenic heterogeneity as a cause of the persistent fraction in HIV-1 neutralization.Res Sq [Preprint]. 2023 Feb 24:rs.3.rs-2613503. doi: 10.21203/rs.3.rs-2613503/v1. Res Sq. 2023. Update in: Retrovirology. 2023 May 27;20(1):9. doi: 10.1186/s12977-023-00624-9 PMID: 36865101 Free PMC article. Updated. Preprint.
-
Mass spectrometry for the identification and analysis of highly complex glycosylation of therapeutic or pathogenic proteins.Expert Rev Proteomics. 2020 Apr;17(4):275-296. doi: 10.1080/14789450.2020.1769479. Epub 2020 May 28. Expert Rev Proteomics. 2020. PMID: 32406805 Free PMC article. Review.
-
Structure-based neural network protein-carbohydrate interaction predictions at the residue level.Front Bioinform. 2023 Jun 20;3:1186531. doi: 10.3389/fbinf.2023.1186531. eCollection 2023. Front Bioinform. 2023. PMID: 37409346 Free PMC article.
-
TLR-9 agonist and CD40-targeting vaccination induces HIV-1 envelope-specific B cells with a diversified immunoglobulin repertoire in humanized mice.PLoS Pathog. 2020 Nov 30;16(11):e1009025. doi: 10.1371/journal.ppat.1009025. eCollection 2020 Nov. PLoS Pathog. 2020. PMID: 33253297 Free PMC article.
-
Synthetic Neoglycoconjugates of Hepta- and Nonamannoside Ligands for Eliciting Oligomannose-Specific HIV-1-Neutralizing Antibodies.Chembiochem. 2022 Apr 5;23(7):e202200061. doi: 10.1002/cbic.202200061. Epub 2022 Feb 11. Chembiochem. 2022. PMID: 35104013 Free PMC article.
References
-
- World Health Assembly . World Health Organisation; Geneva: 1980. Declaration of Global Eradication of Smallpox.
-
- Henao-Restrepo A.M., Camacho A., Longini I.M., Watson C.H., Edmunds W.J., Egger M. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!) Lancet. 2017;389:505–518. - PMC - PubMed
-
- Rerks-Ngarm S., Pitisuttithum P., Nitayaphan S., Kaewkungwal J., Chiu J., Paris R. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

