RNA viruses promote activation of the NLRP3 inflammasome through cytopathogenic effect-induced potassium efflux
- PMID: 31024004
- PMCID: PMC6483999
- DOI: 10.1038/s41419-019-1579-0
RNA viruses promote activation of the NLRP3 inflammasome through cytopathogenic effect-induced potassium efflux
Abstract
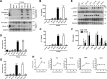
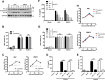
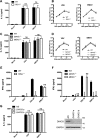
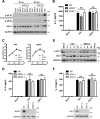
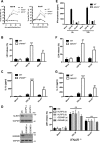
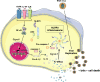
Early detection of viruses by the innate immune system is crucial for host defense. The NLRP3 inflammasome, through activation of caspase-1, promotes the maturation of IL-1β and IL-18, which are critical for antiviral immunity and inflammatory response. However, the mechanism by which viruses activate this inflammasome is still debated. Here, we report that the replication of cytopathogenic RNA viruses such as vesicular stomatitis virus (VSV) or encephalomyocarditis virus (EMCV) induced a lytic cell death leading to potassium efflux, the common trigger of NLRP3 inflammasome activation. This lytic cell death was not prevented by a chemical or genetic inhibition of apoptosis, pyroptosis, or necroptosis but required the viral replication. Hence, the viruses that stimulated type I IFNs production after their sensing did not activate NLRP3 inflammasome due to an inhibition of their replication. In contrast, NLRP3 inflammasome activation induced by RNA virus infection was stimulated in IFNAR-deficient or MAVS-deficient cells consequently to an increased viral replication and ensuing lytic cell death. Therefore, in a context of inefficient IFN response, viral replication-induced lytic cell death activates of the NLRP3 inflammasome to fight against infection.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
The NLRP3 inflammasome detects encephalomyocarditis virus and vesicular stomatitis virus infection.J Virol. 2011 May;85(9):4167-72. doi: 10.1128/JVI.01687-10. Epub 2011 Feb 2. J Virol. 2011. PMID: 21289120 Free PMC article.
-
Encephalomyocarditis virus viroporin 2B activates NLRP3 inflammasome.PLoS Pathog. 2012;8(8):e1002857. doi: 10.1371/journal.ppat.1002857. Epub 2012 Aug 9. PLoS Pathog. 2012. PMID: 22916014 Free PMC article.
-
Sendai Virus V Protein Inhibits the Secretion of Interleukin-1β by Preventing NLRP3 Inflammasome Assembly.J Virol. 2018 Sep 12;92(19):e00842-18. doi: 10.1128/JVI.00842-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021903 Free PMC article.
-
NLRP3 Inflammasome-A Key Player in Antiviral Responses.Front Immunol. 2020 Feb 18;11:211. doi: 10.3389/fimmu.2020.00211. eCollection 2020. Front Immunol. 2020. PMID: 32133002 Free PMC article. Review.
-
The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis).Immunol Rev. 2020 Sep;297(1):26-38. doi: 10.1111/imr.12909. Epub 2020 Jul 29. Immunol Rev. 2020. PMID: 32729116 Free PMC article. Review.
Cited by
-
The genetic advantage of healthy centenarians: unraveling the central role of NLRP3 in exceptional healthspan.Front Aging. 2024 Sep 5;5:1452453. doi: 10.3389/fragi.2024.1452453. eCollection 2024. Front Aging. 2024. PMID: 39301197 Free PMC article. Review.
-
Autophagy and Inflammation: Regulatory Roles in Viral Infections.Biomolecules. 2023 Sep 27;13(10):1454. doi: 10.3390/biom13101454. Biomolecules. 2023. PMID: 37892135 Free PMC article. Review.
-
The involvement of regulated cell death forms in modulating the bacterial and viral pathogenesis.Int Rev Cell Mol Biol. 2020;353:211-253. doi: 10.1016/bs.ircmb.2019.12.008. Epub 2020 Jan 27. Int Rev Cell Mol Biol. 2020. PMID: 32381176 Free PMC article. Review.
-
Novel Coronavirus-Induced NLRP3 Inflammasome Activation: A Potential Drug Target in the Treatment of COVID-19.Front Immunol. 2020 May 19;11:1021. doi: 10.3389/fimmu.2020.01021. eCollection 2020. Front Immunol. 2020. PMID: 32574259 Free PMC article. No abstract available.
-
Virus-Like Cytosolic and Cell-Free Oxidatively Damaged Nucleic Acids Likely Drive Inflammation, Synapse Degeneration, and Neuron Death in Alzheimer's Disease.J Alzheimers Dis Rep. 2023 Jan 9;7(1):1-19. doi: 10.3233/ADR-220047. eCollection 2023. J Alzheimers Dis Rep. 2023. PMID: 36761106 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

