Lack of Middle East Respiratory Syndrome Coronavirus Transmission in Rabbits
- PMID: 31022948
- PMCID: PMC6520746
- DOI: 10.3390/v11040381
Lack of Middle East Respiratory Syndrome Coronavirus Transmission in Rabbits
Abstract
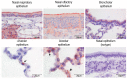
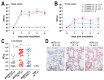
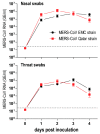
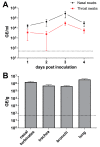
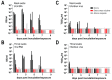
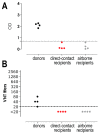
Middle East respiratory syndrome coronavirus (MERS-CoV) transmission from dromedaries to humans has resulted in major outbreaks in the Middle East. Although some other livestock animal species have been shown to be susceptible to MERS-CoV, it is not fully understood why the spread of the virus in these animal species has not been observed in the field. In this study, we used rabbits to further characterize the transmission potential of MERS-CoV. In line with the presence of MERS-CoV receptor in the rabbit nasal epithelium, high levels of viral RNA were shed from the nose following virus inoculation. However, unlike MERS-CoV-infected dromedaries, these rabbits did not develop clinical manifestations including nasal discharge and did shed only limited amounts of infectious virus from the nose. Consistently, no transmission by contact or airborne routes was observed in rabbits. Our data indicate that despite relatively high viral RNA levels produced, low levels of infectious virus are excreted in the upper respiratory tract of rabbits as compared to dromedary camels, thus resulting in a lack of viral transmission.
Keywords: MERS-coronavirus; rabbits; transmission.
Conflict of interest statement
K.S., L.d.W., and G.J.A. are full time employees at Viroclinics Biosciences BV. The other authors have no conflict of interest to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Middle East respiratory syndrome coronavirus experimental transmission using a pig model.Transbound Emerg Dis. 2017 Oct;64(5):1342-1345. doi: 10.1111/tbed.12668. Epub 2017 Jun 26. Transbound Emerg Dis. 2017. PMID: 28653496 Free PMC article.
-
Bactrian camels shed large quantities of Middle East respiratory syndrome coronavirus (MERS-CoV) after experimental infection.Emerg Microbes Infect. 2019;8(1):717-723. doi: 10.1080/22221751.2019.1618687. Emerg Microbes Infect. 2019. PMID: 31119984 Free PMC article.
-
Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels.Emerg Infect Dis. 2014 Dec;20(12):1999-2005. doi: 10.3201/eid2012.141280. Emerg Infect Dis. 2014. PMID: 25418529 Free PMC article.
-
[Dromedary camels and Middle East respiratory syndrome: MERS coronavirus in the 'ship of the desert'].Ned Tijdschr Geneeskd. 2014;158:A7806. Ned Tijdschr Geneeskd. 2014. PMID: 25248734 Review. Dutch.
-
Dromedary Camels and the Transmission of Middle East Respiratory Syndrome Coronavirus (MERS-CoV).Transbound Emerg Dis. 2017 Apr;64(2):344-353. doi: 10.1111/tbed.12401. Epub 2015 Aug 10. Transbound Emerg Dis. 2017. PMID: 26256102 Free PMC article. Review.
Cited by
-
Susceptibility to SARS, MERS, and COVID-19 from animal health perspective.Open Vet J. 2020 Aug;10(2):164-177. doi: 10.4314/ovj.v10i2.6. Epub 2020 May 10. Open Vet J. 2020. PMID: 32821661 Free PMC article. Review.
-
MERS Coronavirus: An Emerging Zoonotic Virus.Viruses. 2019 Jul 19;11(7):663. doi: 10.3390/v11070663. Viruses. 2019. PMID: 31331035 Free PMC article.
-
Species-Specific Colocalization of Middle East Respiratory Syndrome Coronavirus Attachment and Entry Receptors.J Virol. 2019 Jul 30;93(16):e00107-19. doi: 10.1128/JVI.00107-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31167913 Free PMC article.
-
Animal models for the risk assessment of viral pandemic potential.Lab Anim Res. 2020 Apr 22;36:11. doi: 10.1186/s42826-020-00040-6. eCollection 2020. Lab Anim Res. 2020. PMID: 32337177 Free PMC article. Review.
-
Zoonotic and Reverse Zoonotic Transmissibility of SARS-CoV-2.Virus Res. 2021 Sep;302:198473. doi: 10.1016/j.virusres.2021.198473. Epub 2021 Jun 9. Virus Res. 2021. PMID: 34118360 Free PMC article. Review.
References
-
- Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Burbelo P.D., de Wit E., Munster V.J., Hensley L.E., Zalmout I.S., et al. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014;5:e00884-14. doi: 10.1128/mBio.01002-14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

