A cytorhabdovirus phosphoprotein forms mobile inclusions trafficked on the actin/ER network for viral RNA synthesis
- PMID: 31020313
- PMCID: PMC6685698
- DOI: 10.1093/jxb/erz195
A cytorhabdovirus phosphoprotein forms mobile inclusions trafficked on the actin/ER network for viral RNA synthesis
Abstract
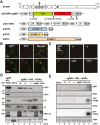
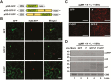
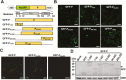
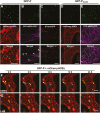
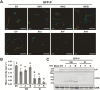
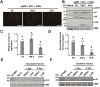
As obligate parasites, plant viruses usually hijack host cytoskeletons for replication and movement. Rhabdoviruses are enveloped, negative-stranded RNA viruses that infect vertebrates, invertebrates, and plants, but the mechanisms of intracellular trafficking of plant rhabdovirus proteins are largely unknown. Here, we used Barley yellow striate mosaic virus (BYSMV), a plant cytorhabdovirus, as a model to investigate the effects of the actin cytoskeleton on viral intracellular movement and viral RNA synthesis in a mini-replicon (MR) system. The BYSMV P protein forms mobile inclusion bodies that are trafficked along the actin/endoplasmic reticulum network, and recruit the N and L proteins into viroplasm-like structures. Deletion analysis showed that the N terminal region (aa 43-55) and the remaining region (aa 56-295) of BYSMV P are essential for the mobility and formation of inclusions, respectively. Overexpression of myosin XI-K tails completely abolishes the trafficking activity of P bodies, and is accompanied by a significant reduction of viral MR RNA synthesis. These results suggest that BYSMV P contributes to the formation and trafficking of viroplasm-like structures along the ER/actin network driven by myosin XI-K. Thus, rhabdovirus P appears to be a dynamic hub protein for efficient recruitment of viral proteins, thereby promoting viral RNA synthesis.
Keywords: Barley yellow striate mosaic virus; Actin cytoskeleton; P bodies; intracellular movement; myosin motor; rhabdovirus.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures

Similar articles
-
CCR4, a RNA decay factor, is hijacked by a plant cytorhabdovirus phosphoprotein to facilitate virus replication.Elife. 2020 Mar 24;9:e53753. doi: 10.7554/eLife.53753. Elife. 2020. PMID: 32207684 Free PMC article.
-
Characterization of the complete genome of Barley yellow striate mosaic virus reveals a nested gene encoding a small hydrophobic protein.Virology. 2015 Apr;478:112-22. doi: 10.1016/j.virol.2014.12.042. Epub 2015 Feb 7. Virology. 2015. PMID: 25666524
-
Host casein kinase 1-mediated phosphorylation modulates phase separation of a rhabdovirus phosphoprotein and virus infection.Elife. 2022 Feb 22;11:e74884. doi: 10.7554/eLife.74884. Elife. 2022. PMID: 35191833 Free PMC article.
-
Actin-myosin XI: an intracellular control network in plants.Biochem Biophys Res Commun. 2018 Nov 25;506(2):403-408. doi: 10.1016/j.bbrc.2017.12.169. Epub 2018 Jan 5. Biochem Biophys Res Commun. 2018. PMID: 29307817 Review.
-
Structural insights into the rhabdovirus transcription/replication complex.Virus Res. 2011 Dec;162(1-2):126-37. doi: 10.1016/j.virusres.2011.09.025. Epub 2011 Sep 22. Virus Res. 2011. PMID: 21963663 Review.
Cited by
-
A small peptide inhibits siRNA amplification in plants by mediating autophagic degradation of SGS3/RDR6 bodies.EMBO J. 2021 Aug 2;40(15):e108050. doi: 10.15252/embj.2021108050. Epub 2021 Jun 22. EMBO J. 2021. PMID: 34155657 Free PMC article.
-
A plant cytorhabdovirus modulates locomotor activity of insect vectors to enhance virus transmission.Nat Commun. 2023 Sep 16;14(1):5754. doi: 10.1038/s41467-023-41503-3. Nat Commun. 2023. PMID: 37717061 Free PMC article.
-
Development of a Mini-Replicon-Based Reverse-Genetics System for Rice Stripe Tenuivirus.J Virol. 2021 Jun 24;95(14):e0058921. doi: 10.1128/JVI.00589-21. Epub 2021 Jun 24. J Virol. 2021. PMID: 33952642 Free PMC article.
-
CCR4, a RNA decay factor, is hijacked by a plant cytorhabdovirus phosphoprotein to facilitate virus replication.Elife. 2020 Mar 24;9:e53753. doi: 10.7554/eLife.53753. Elife. 2020. PMID: 32207684 Free PMC article.
-
The plant rhabdovirus viroporin P9 facilitates insect-mediated virus transmission in barley.Plant Cell. 2024 Sep 3;36(9):3483-3497. doi: 10.1093/plcell/koae162. Plant Cell. 2024. PMID: 38819305
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

