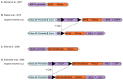
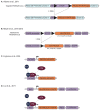
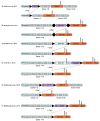
Mouse Models for Exploring the Biological Consequences and Clinical Significance of PIK3CA Mutations
- PMID: 31018529
- PMCID: PMC6523081
- DOI: 10.3390/biom9040158
Mouse Models for Exploring the Biological Consequences and Clinical Significance of PIK3CA Mutations
Abstract
The phosphatidylinositol 3-kinase (PI3K) pathway is involved in a myriad of cellular signalling pathways that regulate cell growth, metabolism, proliferation and survival. As a result, alterations in the PI3K pathway are frequently associated with human cancers. Indeed, PIK3CA-the gene encoding the p110α catalytic subunit of PI3K-is one of the most commonly mutated human oncogenes. PIK3CA mutations have also been implicated in non-malignant conditions including congenital overgrowth syndromes and vascular malformations. In order to study the role of PIK3CA mutations in driving tumorigenesis and tissue overgrowth and to test potential therapeutic interventions for these conditions, model systems are essential. In this review we discuss the various mouse models currently available for preclinical studies into the biological consequences and clinical significance of PIK3CA mutations.
Keywords: PI 3-kinase; PI3K; PIK3CA; PROS; cancer; knock-in; mouse model; overgrowth; transgenic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Heterozygous expression of the oncogenic Pik3ca(H1047R) mutation during murine development results in fatal embryonic and extraembryonic defects.Dev Biol. 2015 Aug 1;404(1):14-26. doi: 10.1016/j.ydbio.2015.04.022. Epub 2015 May 7. Dev Biol. 2015. PMID: 25958091
-
Mouse models of PIK3CA mutations: one mutation initiates heterogeneous mammary tumors.FEBS J. 2013 Jun;280(12):2758-65. doi: 10.1111/febs.12175. Epub 2013 Mar 1. FEBS J. 2013. PMID: 23384338 Review.
-
Oncogenic mutations of PIK3CA in human cancers.Cell Cycle. 2004 Oct;3(10):1221-4. doi: 10.4161/cc.3.10.1164. Epub 2004 Oct 12. Cell Cycle. 2004. PMID: 15467468
-
PIK3CA hotspot mutations differentially impact responses to MET targeting in MET-driven and non-driven preclinical cancer models.Mol Cancer. 2017 May 22;16(1):93. doi: 10.1186/s12943-017-0660-5. Mol Cancer. 2017. PMID: 28532501 Free PMC article.
-
Gene of the month: PIK3CA.J Clin Pathol. 2015 Apr;68(4):253-7. doi: 10.1136/jclinpath-2015-202885. Epub 2015 Feb 16. J Clin Pathol. 2015. PMID: 25688137 Review.
Cited by
-
Organismal metabolism regulates the expansion of oncogenic PIK3CA mutant clones in normal esophagus.Nat Genet. 2024 Oct;56(10):2144-2157. doi: 10.1038/s41588-024-01891-8. Epub 2024 Aug 21. Nat Genet. 2024. PMID: 39169259 Free PMC article.
-
PIK3CA regulates development of diabetes retinopathy through the PI3K/Akt/mTOR pathway.PLoS One. 2024 Jan 9;19(1):e0295813. doi: 10.1371/journal.pone.0295813. eCollection 2024. PLoS One. 2024. PMID: 38194422 Free PMC article.
-
Network Pharmacology-Based Exploration on the Intervention of Qinghao Biejia Decoction on the Inflammation-Carcinoma Transformation Process of Chronic Liver Disease via MAPK and PI3k/AKT Pathway.Biomed Res Int. 2022 Oct 14;2022:9202128. doi: 10.1155/2022/9202128. eCollection 2022. Biomed Res Int. 2022. PMID: 36277879 Free PMC article.
-
PI3K inhibitors are finally coming of age.Nat Rev Drug Discov. 2021 Oct;20(10):741-769. doi: 10.1038/s41573-021-00209-1. Epub 2021 Jun 14. Nat Rev Drug Discov. 2021. PMID: 34127844 Free PMC article. Review.
-
Human Colorectal Cancer from the Perspective of Mouse Models.Genes (Basel). 2019 Oct 11;10(10):788. doi: 10.3390/genes10100788. Genes (Basel). 2019. PMID: 31614493 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

