A bypass mechanism of abiraterone-resistant prostate cancer: Accumulating CYP17A1 substrates activate androgen receptor signaling
- PMID: 31017696
- PMCID: PMC6593470
- DOI: 10.1002/pros.23799
A bypass mechanism of abiraterone-resistant prostate cancer: Accumulating CYP17A1 substrates activate androgen receptor signaling
Abstract
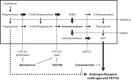
Background: Intratumoral steroidogenesis and its potential relevance in castration-resistant prostate cancer (CRPC) and in cytochrome P450, family 17, subfamily A, polypeptide 1 (CYP17A1)-inhibitor treated hormone-naïve and patients with CRPC are not well established. In this study, we tested if substrates for de novo steroidogenesis accumulating during CYP17A1 inhibition may drive cell growth in relevant preclinical models.
Methods: PCa cell lines and their respective CRPC sublines were used to model CRPC in vitro. Precursor steroids pregnenolone (Preg) and progesterone (Prog) served as substrate for de novo steroid synthesis. TAK700 (orteronel), abiraterone, and small interfering RNA (siRNA) against CYP17A1 were used to block CYP17A1 enzyme activity. The antiandrogen RD162 was used to assess androgen receptor (AR) involvement. Cell growth was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. AR-target gene expression was quantified by reverse transcription polymerase chain reaction (RT-PCR). Nuclear import studies using cells with green fluorescent protein (GFP)-tagged AR were performed to assess the potential of precursor steroids to directly activate AR.
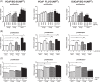
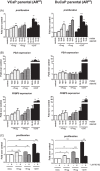
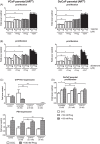
Results: Preg and Prog stimulated cell proliferation and AR target gene expression in VCaP, DuCaP, LNCaP, and their respective CRPC sublines. The antiandrogen RD162, but not CYP17A1 inhibition with TAK700, abiraterone or siRNA, was able to block Preg- and Prog-induced proliferation. In contrast to TAK700, abiraterone also affected dihydrotestosterone-induced cell growth, indicating direct AR binding. Furthermore, Prog-induced AR translocation was not affected by treatment with TAK700 or abiraterone, while it was effectively blocked by the AR antagonist enzalutamide, further demonstrating the direct AR activation by Prog.
Conclusion: Activation of the AR by clinically relevant levels of Preg and Prog accumulating in abiraterone-treated patients may act as a driver for CRPC. These data provide a scientific rationale for combining CYP17A1 inhibitors with antiandrogens, particularly in patients with overexpressed or mutated-AR.
Keywords: TAK700; abiraterone resistance; androgen receptor activation; castration-resistant prostate cancer; cytochrome P450, family 17, subfamily A, polypeptide 1 inhibitor.
© 2019 The Authors. The Prostate Published by Wiley Periodicals, Inc.
Conflict of interest statement
RW receives consultancy and speaker fees from Sanofi, Millennium, Merck, Roche. RS receives speaker fee from Sanofi, Janssen Pharmaceuticals. WW receives grant supports from Sanofi, Millennium, Janssen Pharmaceuticals, Servier.
Figures

Similar articles
-
Abiraterone switches castration-resistant prostate cancer dependency from adrenal androgens towards androgen receptor variants and glucocorticoid receptor signalling.Prostate. 2022 Apr;82(5):505-516. doi: 10.1002/pros.24297. Epub 2022 Jan 17. Prostate. 2022. PMID: 35037287 Free PMC article.
-
Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors.Cancer Res. 2011 Oct 15;71(20):6503-13. doi: 10.1158/0008-5472.CAN-11-0532. Epub 2011 Aug 25. Cancer Res. 2011. PMID: 21868758 Free PMC article.
-
Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis.Oncogene. 2014 May 29;33(22):2815-25. doi: 10.1038/onc.2013.235. Epub 2013 Jun 10. Oncogene. 2014. PMID: 23752196 Free PMC article. Review.
-
Targeting of CYP17A1 Lyase by VT-464 Inhibits Adrenal and Intratumoral Androgen Biosynthesis and Tumor Growth of Castration Resistant Prostate Cancer.Sci Rep. 2016 Oct 17;6:35354. doi: 10.1038/srep35354. Sci Rep. 2016. PMID: 27748439 Free PMC article.
-
Exploitation of the Androgen Receptor to Overcome Taxane Resistance in Advanced Prostate Cancer.Adv Cancer Res. 2015;127:123-58. doi: 10.1016/bs.acr.2015.03.001. Epub 2015 Mar 29. Adv Cancer Res. 2015. PMID: 26093899 Review.
Cited by
-
Abiraterone switches castration-resistant prostate cancer dependency from adrenal androgens towards androgen receptor variants and glucocorticoid receptor signalling.Prostate. 2022 Apr;82(5):505-516. doi: 10.1002/pros.24297. Epub 2022 Jan 17. Prostate. 2022. PMID: 35037287 Free PMC article.
-
Androgen Receptor Signaling Inhibition in Advanced Castration Resistance Prostate Cancer: What Is Expected for the Near Future?Cancers (Basel). 2022 Dec 9;14(24):6071. doi: 10.3390/cancers14246071. Cancers (Basel). 2022. PMID: 36551557 Free PMC article. Review.
-
Apalutamide Sensitizes Prostate Cancer to Ionizing Radiation via Inhibition of Non-Homologous End-Joining DNA Repair.Cancers (Basel). 2019 Oct 18;11(10):1593. doi: 10.3390/cancers11101593. Cancers (Basel). 2019. PMID: 31635359 Free PMC article.
-
Cell Line Characteristics Predict Subsequent Resistance to Androgen Receptor-Targeted Agents (ARTA) in Preclinical Models of Prostate Cancer.Front Oncol. 2022 Jun 13;12:877613. doi: 10.3389/fonc.2022.877613. eCollection 2022. Front Oncol. 2022. PMID: 35769712 Free PMC article.
-
Abiraterone Acetate Induces CREB1 Phosphorylation and Enhances the Function of the CBP-p300 Complex, Leading to Resistance in Prostate Cancer Cells.Clin Cancer Res. 2021 Apr 1;27(7):2087-2099. doi: 10.1158/1078-0432.CCR-20-4391. Epub 2021 Jan 25. Clin Cancer Res. 2021. PMID: 33495313 Free PMC article.
References
-
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187‐97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

